10 21c How To Find The Intermolecular Forces In Ch3ch2cl C2h5cl

Intermolecular Forces In Chemistry 10.21c | how to find the intermolecular forces in ch3ch2cl (c2h5cl) identify the intermolecular forces present in the following solids:ch3ch2cl (c2h5cl)openstax™ is a. In ch₃ch₂cl, the intermolecular forces present are london dispersion forces and dipole dipole interactions. however, it does not exhibit hydrogen bonding. thus, the correct options are a (induced dipole induced dipole) and b (dipole dipole).

Solved Intermolecular Forces List All Of The Kinds Of Chegg The forces involved are dipole dipole attractions and dispersion (induced dipole) forces. ch3cl has a larger dipole (1.892 d) than ch3i, therefore one might expect ch3cl to have stronger intermolecular forces as a liquid. Chloroethane (ch3ch2cl) experiences both london dispersion forces (ldfs), present in all molecules, and dipole dipole forces due to the molecule's polarity stemming from the polar c cl bond. Examples intermolecular forces from lewis structures (openchem) [ "article:topic", "openchem", "dipole dipole", "hydrogen bonding", "dispersion", "showtoc:no", "license:ccbyncsa", "licenseversion:40" ]. Identify the intermolecular forces present in ch3ch2cl. i. hydrogen bonding ii. dipole dipole attraction iii. dispersion forces iv. none of the above. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on.

10 10 Pts Identify The Intermolecular Forces Present In The Examples intermolecular forces from lewis structures (openchem) [ "article:topic", "openchem", "dipole dipole", "hydrogen bonding", "dispersion", "showtoc:no", "license:ccbyncsa", "licenseversion:40" ]. Identify the intermolecular forces present in ch3ch2cl. i. hydrogen bonding ii. dipole dipole attraction iii. dispersion forces iv. none of the above. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. Ch3ch2cl is a relatively small molecule with a moderate number of electrons, so dispersion forces are present. therefore, the correct answer is ii and iii: dipole dipole attraction and dispersion forces. In ch₃ch₂cl (chloroethane), the main intermolecular forces are dipole dipole forces, due to the polarity of the molecule, and london dispersion forces, which are present in all molecules. "identify the intermolecular forces present in the following solids:ch3ch2cl (c2h5cl)"**intermolecular forces in ch3ch2cl (c2h5cl):**1. **dipole dipole inter. Identify the intermolecular forces present in the following solids: ch3ch2oh (c2h5oh) more.
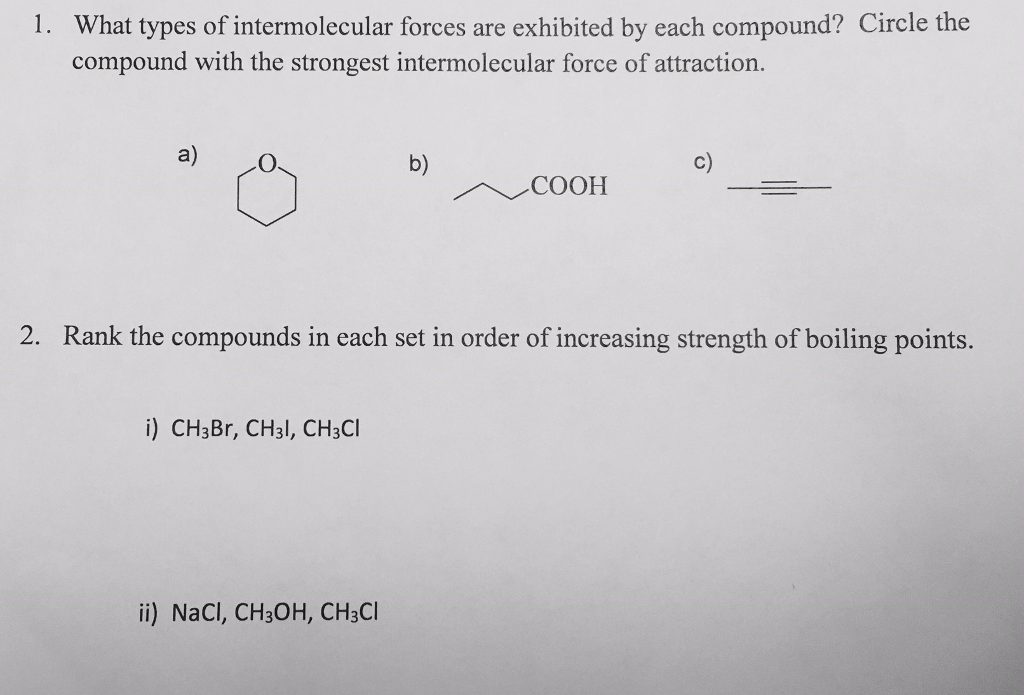
Solved What Types Of Intermolecular Forces Are Exhibited By Chegg Ch3ch2cl is a relatively small molecule with a moderate number of electrons, so dispersion forces are present. therefore, the correct answer is ii and iii: dipole dipole attraction and dispersion forces. In ch₃ch₂cl (chloroethane), the main intermolecular forces are dipole dipole forces, due to the polarity of the molecule, and london dispersion forces, which are present in all molecules. "identify the intermolecular forces present in the following solids:ch3ch2cl (c2h5cl)"**intermolecular forces in ch3ch2cl (c2h5cl):**1. **dipole dipole inter. Identify the intermolecular forces present in the following solids: ch3ch2oh (c2h5oh) more.

Diagram Ch3och3 Intermolecular Forces Diagram Mydiagram Online "identify the intermolecular forces present in the following solids:ch3ch2cl (c2h5cl)"**intermolecular forces in ch3ch2cl (c2h5cl):**1. **dipole dipole inter. Identify the intermolecular forces present in the following solids: ch3ch2oh (c2h5oh) more.
Comments are closed.