
Solubility Rules Pdf Pdf Solubility Chemical Compounds Chemistry 100 unit 3 pages 5vand 6.all about the solubility rules. The solubility rules have multiple uses, including predicting whether a chemical will dissolve, predicting precipitate formation, and purifying samples. to use the solubility rules, check the anion (the negative part of the ion) and see whether it is soluble or insoluble.

Solubility Rules Solubility Of Common Ionic Compounds Printerview When a substance is mixed with a solvent, there are several possible results. the determining factor for the result is the solubility of the substance, which is defined as the maximum possible concentration of the solute. the solubility rules help determine which substances are soluble, and to what extent. The solubility rules. if the anion is no 3 , hco 3 , c 2 h 3 o 2 , clo 3 or clo 4 , the compound is soluble (aq) ; if the cation is an alkali metal cation (li , na , k , rb , cs ) or nh 4 , the compound is soluble (aq) ; if the anion is cl , br or i , the compound is soluble (aq) except for agcl, hg 2 cl 2 and pbcl 2 .; if the anion is so 4 2 , the compound is soluble (aq) except for. In this article, we look at the common solubility rules of chemistry, which state which anions and cations are usually soluble, and which aren’t. we will also display a solubility chart that states the solubility of many common ionic compounds. All nitrates are soluble. all chlorides are soluble except: agcl, pbcl2, and hg2cl2. all sulfates are soluble except: ag2so4, pbso4, hg2so4, caso4, srso4, and baso4. the carbonate of group 1 metals and (nh4)2co3 are soluble. all other carbonates are insoluble.
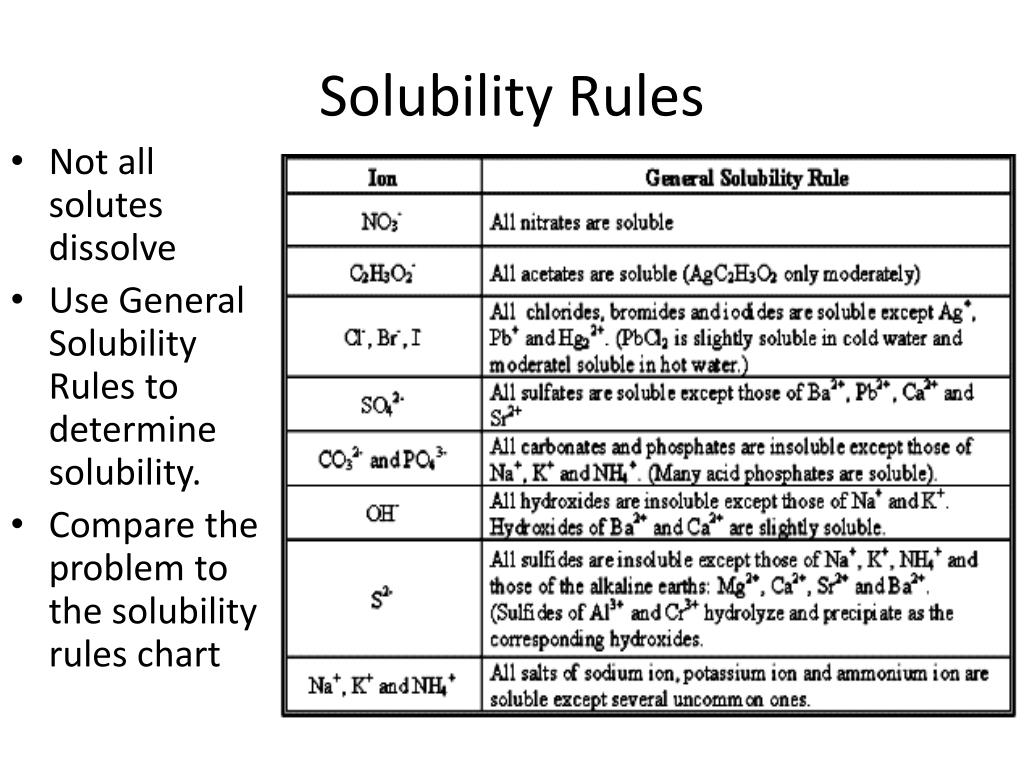
Solubility Chart And Solubility Rules 49 Off In this article, we look at the common solubility rules of chemistry, which state which anions and cations are usually soluble, and which aren’t. we will also display a solubility chart that states the solubility of many common ionic compounds. All nitrates are soluble. all chlorides are soluble except: agcl, pbcl2, and hg2cl2. all sulfates are soluble except: ag2so4, pbso4, hg2so4, caso4, srso4, and baso4. the carbonate of group 1 metals and (nh4)2co3 are soluble. all other carbonates are insoluble. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. our article on solubility explains how ionic compounds dissolve in water to form a solution. however, to predict whether the compound will dissolve or not, we need to know a few general rules. The solubility rules 1. the nitrates, chlorates, and acetates of all metals are soluble in water. silver acetate is sparingly soluble. 2. all sodium, potassium, and ammonium salts are soluble in water. 3. the chlorides, bromides, and iodides of all metals except lead, silver, and mercury(i) are soluble in water. hgi2 is insoluble in water. Csus chemistry 1a solubility rules dr. mack the following is a list that complies the general solubility rules for inorganic compounds (salts). there are some rare exceptions that are not covered by this list, they are of no concern to you at this time. Understanding solubility rules is key in chemistry, helping predict how substances behave in water. these rules apply to various compounds, including alkali metals and ammonium, guiding reactions in ap chemistry, honors chemistry, and intro to chemistry.

Solubility Rules Diagram Quizlet When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. our article on solubility explains how ionic compounds dissolve in water to form a solution. however, to predict whether the compound will dissolve or not, we need to know a few general rules. The solubility rules 1. the nitrates, chlorates, and acetates of all metals are soluble in water. silver acetate is sparingly soluble. 2. all sodium, potassium, and ammonium salts are soluble in water. 3. the chlorides, bromides, and iodides of all metals except lead, silver, and mercury(i) are soluble in water. hgi2 is insoluble in water. Csus chemistry 1a solubility rules dr. mack the following is a list that complies the general solubility rules for inorganic compounds (salts). there are some rare exceptions that are not covered by this list, they are of no concern to you at this time. Understanding solubility rules is key in chemistry, helping predict how substances behave in water. these rules apply to various compounds, including alkali metals and ammonium, guiding reactions in ap chemistry, honors chemistry, and intro to chemistry.
