
100u3p9molarity Youtube Chem 100 unit 3 p9 about molarity calculations. This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration (or recalculating grams per ml to moles). you can also calculate the mass of a substance needed to achieve a desired molarity. this article will provide you with the molarity definition and the molarity formula to understand the topic as a whole, you will want to learn the mole.

5piece Tqp3m9009 3m9009 Sot 89 Jpg How is the molarity of a percentage solution calculated? using 70% concentrated nitric acid as an example: 70% nitric acid means that 100 grams of this acid contains 70 grams of hno 3.the concentration is expressed at 70% wt. wt. or 70 wt. % hno 3.some. Molarity. let's recall some definitions: a solution is a mixture where the ratio of solute to solvent remains the same throughout the solution (a homogeneous mixture or mixture with uniform composition). the solvent is the chemical that is present in the larger amount, and the solute is the chemical that is present in the smaller amount molarity or molar concentration is the number of moles. 本页面最后修订于2024年5月30日 (星期四) 21:44。 本站的全部文字在知识共享 署名 相同方式共享 4.0协议之条款下提供,附加条款亦可能应用。 (请参阅使用条款) ®和维基百科标志是维基媒体基金会的注册商标;维基™是维基媒体基金会的商标。. Choose a dna, rna, qpcr calculator from neb, a leader in production and supply of reagents for the life science industry.

유니플러스 本页面最后修订于2024年5月30日 (星期四) 21:44。 本站的全部文字在知识共享 署名 相同方式共享 4.0协议之条款下提供,附加条款亦可能应用。 (请参阅使用条款) ®和维基百科标志是维基媒体基金会的注册商标;维基™是维基媒体基金会的商标。. Choose a dna, rna, qpcr calculator from neb, a leader in production and supply of reagents for the life science industry. Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in particular, of a solute in a solution, in terms of amount of substance per unit volume of solution. in chemistry, the most commonly used unit for molarity is the number of moles per liter, having the unit symbol mol l or mol dm 3 in si units. Protocols, applications and pricing for hplc columns, micro dialysis membranes and kits, 96 well dialysis or equilibrium dialysis plates, and micro sample spe tips for lc ms desalting. Molarity equation. as shown below, the molarity of a solution is defined as the ratio of the molar amount of solute that is present in a solution, relative to the volume of the solution, as a whole. recall that the variable that is utilized to represent the molar quantity of a substance is "n." because, in contrast to the concentrations that have been discussed in the previous sections of this.
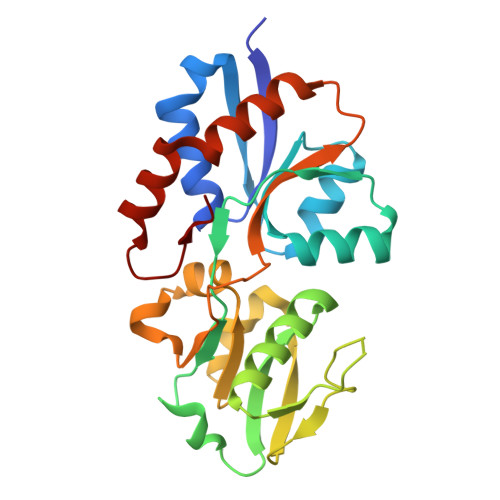
Rcsb Pdb 3up9 Crystal Structure Of A Putative Lipoprotein Actodo Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in particular, of a solute in a solution, in terms of amount of substance per unit volume of solution. in chemistry, the most commonly used unit for molarity is the number of moles per liter, having the unit symbol mol l or mol dm 3 in si units. Protocols, applications and pricing for hplc columns, micro dialysis membranes and kits, 96 well dialysis or equilibrium dialysis plates, and micro sample spe tips for lc ms desalting. Molarity equation. as shown below, the molarity of a solution is defined as the ratio of the molar amount of solute that is present in a solution, relative to the volume of the solution, as a whole. recall that the variable that is utilized to represent the molar quantity of a substance is "n." because, in contrast to the concentrations that have been discussed in the previous sections of this.

Effects Of La0 9sr0 1ga0 9mg0 1o3 δ On The Microstructure And Ionic Molarity equation. as shown below, the molarity of a solution is defined as the ratio of the molar amount of solute that is present in a solution, relative to the volume of the solution, as a whole. recall that the variable that is utilized to represent the molar quantity of a substance is "n." because, in contrast to the concentrations that have been discussed in the previous sections of this.
