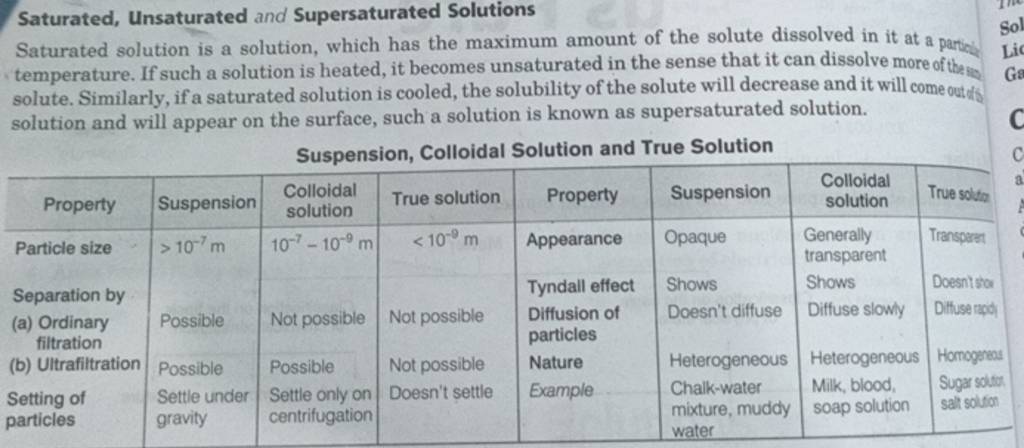
54 Explain Unsaturated Solution Saturated Solutions And Supersaturated Define saturated. define unsaturated. apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. define supersaturated. explain how supersaturated solutions are created. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: saturated, unsaturated, and supersaturated. saturated solution: a saturated solution is a solution in which the maximum amount of solute has been dissolved in a given amount of solvent at a particular temperature.

Unsaturated Saturated And Supersaturated Solutions Science standard when you have an unsaturated solution less solute then the solution is capable of dissolving is mixed in. with a saturated solution there is so much solute present that if you. Unsaturated solution: contains less solute than a saturated solution which completely dissolves leaving no remaining substances. supersaturated solution: a solution that contains more solute than the solvent is capable of dissolving. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Saturated, unsaturated and supersaturated refer to three different conditions of a solution. a saturated solution contains the maximum amount of solute that will dissolve at that temperature. any further addition of solute will result in undissolved solid on the bottom of the container.

Saturated Unsaturated And Supersaturated Solution Chemistry Go It A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Saturated, unsaturated and supersaturated refer to three different conditions of a solution. a saturated solution contains the maximum amount of solute that will dissolve at that temperature. any further addition of solute will result in undissolved solid on the bottom of the container. The libretexts libraries are powered by nice cxone expert and are supported by the department of education open textbook pilot project, the uc davis office of the provost, the uc davis library, the california state university affordable learning solutions program, and merlot. we also acknowledge previous national science foundation support under grant numbers 1246120, 1525057, and 1413739. Given scenarios, graphs, diagrams, or illustrations, the student will determine the type of solution such as saturated, supersaturated, or unsaturated. Unsaturated solution is a solution where more solute can be added to the solution at a constant temperature. in other words, there is still room for more solute to dissolve in the solvent. unsaturated solutions can be created by adding less solute than the solubility limit or by lowering the temperature, which reduces the solubility of the solute. A solution in which the amount of solute is equal to the solute’s solubility is called a saturated solution. a solution in which the amount of solute greater than the solute’s is called a supersaturated solution.

Saturated Supersaturated Unsaturated Solution Over 16 Royalty Free The libretexts libraries are powered by nice cxone expert and are supported by the department of education open textbook pilot project, the uc davis office of the provost, the uc davis library, the california state university affordable learning solutions program, and merlot. we also acknowledge previous national science foundation support under grant numbers 1246120, 1525057, and 1413739. Given scenarios, graphs, diagrams, or illustrations, the student will determine the type of solution such as saturated, supersaturated, or unsaturated. Unsaturated solution is a solution where more solute can be added to the solution at a constant temperature. in other words, there is still room for more solute to dissolve in the solvent. unsaturated solutions can be created by adding less solute than the solubility limit or by lowering the temperature, which reduces the solubility of the solute. A solution in which the amount of solute is equal to the solute’s solubility is called a saturated solution. a solution in which the amount of solute greater than the solute’s is called a supersaturated solution.
Saturated Unsaturated And Supersaturated Solutions Saturated Solution Is Unsaturated solution is a solution where more solute can be added to the solution at a constant temperature. in other words, there is still room for more solute to dissolve in the solvent. unsaturated solutions can be created by adding less solute than the solubility limit or by lowering the temperature, which reduces the solubility of the solute. A solution in which the amount of solute is equal to the solute’s solubility is called a saturated solution. a solution in which the amount of solute greater than the solute’s is called a supersaturated solution.
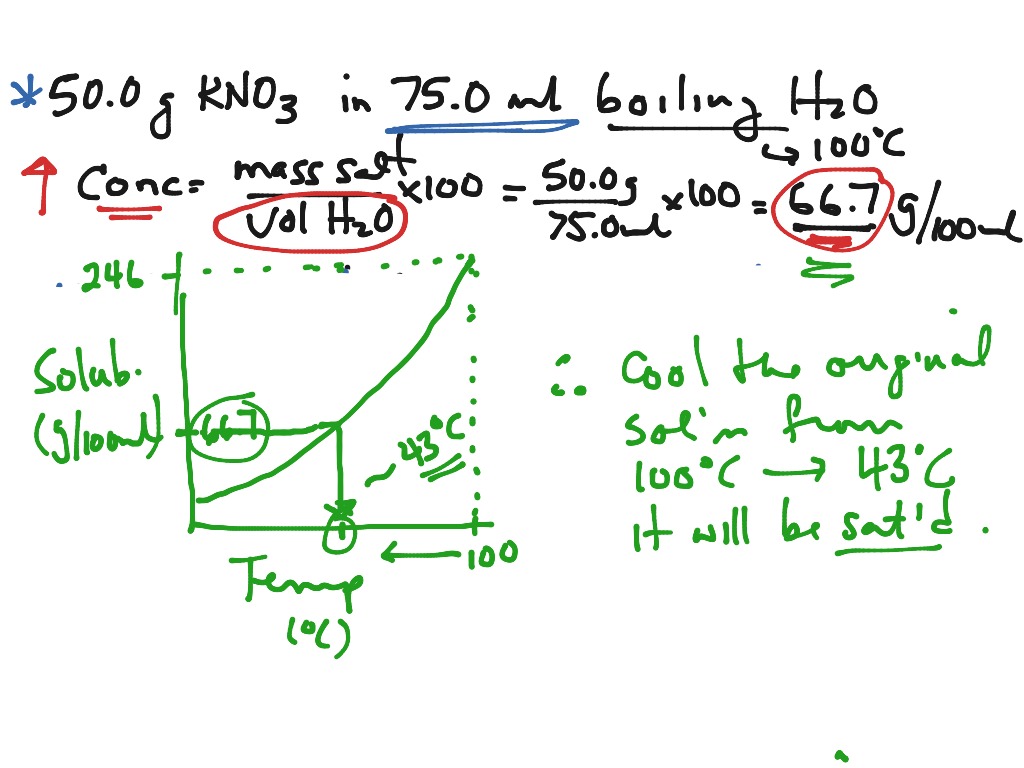
Showme Saturated Unsaturated And Supersaturated Solution
