
Passage 2 Na Hyoa C03 B So3 C Nay 12 D Ag Salt E Complex 1 The You'll get a detailed solution from a subject matter expert that helps you learn core concepts. a) [co(n3)(nh3)5]so4. let's tackle each one step by step. ### a) [co (n3) (nh3)5]so4 ** show more… adi s and 66 other chemistry 101 educators are ready to help you. what is the coordination number of the metal ion in: a. [fe (co)5] b. A) [co(n3)(nh3)5]so4 b) [co(co3)(en)2]cl c) na[alcl4] d) [ag(nh3)2][bf4] your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on.
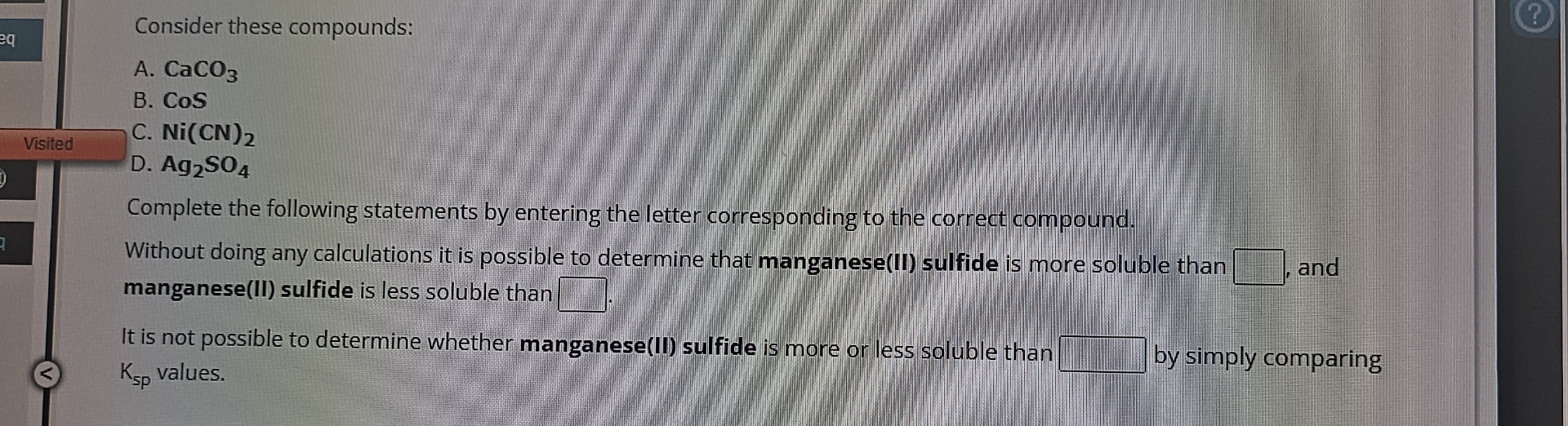
Solved Consider These Compounds A Caco3b Chegg Sodium tetrachloroaluminate is a chemical compound with the formula na al cl 4. it is the sodium salt of the tetrachloroaluminate anion. it was discovered by friedrich wöhler in 1827. [1]: 228. sodium tetrachloroaluminate can be prepared from sodium chloride and aluminium trichloride. Herein, the new mechanochemically prepared orthorhombic naalcl 4 is demonstrated to exhibit a 10 fold enhancement in na conductivity (3.9 × 10 –6 s cm –1 at 30 °c) compared to annealed samples. the feasibility of naalcl 4 for asnbs is also validated for the first time. A method for the preparation of high purity naalcl 4 includes charging a feed to a reactor at an initial temperature less than about 50° c., carrying out a solid state reaction to form a solid. Follow these links to do a live 2d search or do a live 3d search for this compound, sorted by annotation score. this section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.

Figure 1 From Mechanism Of Reaction In Naalcl4 Molten Salt Batteries A method for the preparation of high purity naalcl 4 includes charging a feed to a reactor at an initial temperature less than about 50° c., carrying out a solid state reaction to form a solid. Follow these links to do a live 2d search or do a live 3d search for this compound, sorted by annotation score. this section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here. The coordination complex $$ [co (nh 3) 5 (ono)]^ {2 }$$ consists of a cobalt ion surrounded by five ammonia ligands and one nitrito ligand, where the nitrito is bound through an oxygen atom. (a) co2, (b) nacl, (c) n2o, (d) caco3, (e) nahco3, (f) nh3, (g) h2o, (h) mg (oh)2, (i) mgso4. to solve this problem, we need to match each common name with its corresponding chemical formula. (a) **dry ice:** the common name for solid carbon dioxide is dry ice. its chemical formula is co 2. Naalcl4 crystallizes in the orthorhombic p2 12 12 1 space group. the structure is three dimensional. na1 is bonded in a 7 coordinate geometry to seven cl1 atoms. there are a spread of na–cl bond distances ranging from 2.83–3.46 Å. al3 is bonded in a tetrahedral geometry to four cl1 atoms. Magic angle spinning nuclear magnetic resonance spectroscopy confirms the formation of al 2 o 3 and the oxychloride phases at the interface and sheds insights into the origin of the enhanced ionic conductivity of the composite electrolyte.
