
Quantum Theory Atomic Structure Pdf Atomic Orbital Energy Level Master atomic structure with these 9 build an atom steps, designed to simplify complex concepts like protons, neutrons, electrons, and energy levels. this guide explores atomic models, subatomic particles, and orbital configurations, making it ideal for students and educators. enhance your understanding of chemistry fundamentals and ace your studies with this comprehensive, step by step approach. Quantum numbers and atomic orbitals each electron in an atom is described by four different quantum numbers (n, l, m l, & m s) 1.principal quantum number (n): specifies the energy of an electron and the size of the orbital. all orbitals that have the same value of n are said to be in the same shell (level). n takes the values of 1, 2, 3, …, ∞.

An In Depth Exploration Of Atomic Structure And Quantum Theory Pdf These quantum numbers describe the size, shape, and orientation in space of the orbitals on an atom. the principal quantum number (n) describes the size of the orbital. orbitals for which n = 2 are larger than those for which n = 1, for example. because they have opposite electrical charges, electrons are attracted to the nucleus of the atom. Understanding quantum numbers, atomic orbitals, and electron configurations is essential for explaining electron properties and behaviors in atoms. quantum numbers like principal, angular momentum, magnetic, and spin numbers characterize electrons. Describe the structure of shells and subshells in multi electron atoms, and relate them to the observed emission spectra. explain the bohr sommerfeld model. for an electron in a given subshell, determine the quantum numbers, and explain your answer. use the rydberg equation to calculate the values of hydrogen spectral lines. Having introduced the basics of atomic structure and quantum mechanics, in chapter 3, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. this allows us to determine which orbitals are occupied by electrons in each atom.

Atomic Orbitals And Quantum Numbers Understanding The Building Describe the structure of shells and subshells in multi electron atoms, and relate them to the observed emission spectra. explain the bohr sommerfeld model. for an electron in a given subshell, determine the quantum numbers, and explain your answer. use the rydberg equation to calculate the values of hydrogen spectral lines. Having introduced the basics of atomic structure and quantum mechanics, in chapter 3, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. this allows us to determine which orbitals are occupied by electrons in each atom. Each electron in an atom is described by four different quantum numbers. the first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. 1. principal quantum number (n): n = 1, 2, 3, …, 8. In this activity you will acquire an ability to write electron configurations, orbital notations and a set of quantum numbers for electrons within elements on the periodic table. you will also be able to justify oxidation or valence states using electron configurations and orbital notations. Quantum numbers. the solutions of the schrödinger mathematical equations, known as wave functions, provide us with a set of quantum numbers. these quantum numbers describe the location, energy, and orientation in space of the electrons within an atom. one of these, the principal quantum number (n) is analogous to bohr's principal energy levels. Lecture 06 2022 04 08 pg. 1 2022 04 08 chem 14a lecture 6: atomic orbitals and quantum numbers textbook readings: focus 1d (skip 1d.1 and 1d.2) suggested textbook problems: 1d.1 2, 9,11 26 objective: • know the 4 quantum numbers for atomic orbitals: the allowed values and what it represents • know the shape of the atomic orbitals • know.

The Quantum Numbers Understanding Atomic Structure Each electron in an atom is described by four different quantum numbers. the first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. 1. principal quantum number (n): n = 1, 2, 3, …, 8. In this activity you will acquire an ability to write electron configurations, orbital notations and a set of quantum numbers for electrons within elements on the periodic table. you will also be able to justify oxidation or valence states using electron configurations and orbital notations. Quantum numbers. the solutions of the schrödinger mathematical equations, known as wave functions, provide us with a set of quantum numbers. these quantum numbers describe the location, energy, and orientation in space of the electrons within an atom. one of these, the principal quantum number (n) is analogous to bohr's principal energy levels. Lecture 06 2022 04 08 pg. 1 2022 04 08 chem 14a lecture 6: atomic orbitals and quantum numbers textbook readings: focus 1d (skip 1d.1 and 1d.2) suggested textbook problems: 1d.1 2, 9,11 26 objective: • know the 4 quantum numbers for atomic orbitals: the allowed values and what it represents • know the shape of the atomic orbitals • know.
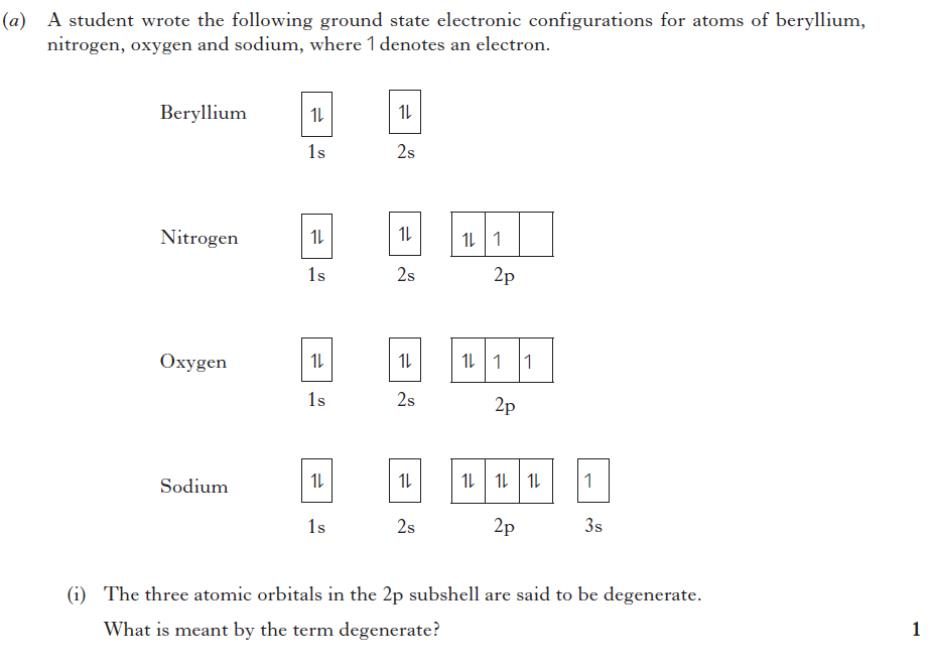
Exercise 1 2b Atomic Orbitals Quantum Numbers Ah Inorganic Physical Quantum numbers. the solutions of the schrödinger mathematical equations, known as wave functions, provide us with a set of quantum numbers. these quantum numbers describe the location, energy, and orientation in space of the electrons within an atom. one of these, the principal quantum number (n) is analogous to bohr's principal energy levels. Lecture 06 2022 04 08 pg. 1 2022 04 08 chem 14a lecture 6: atomic orbitals and quantum numbers textbook readings: focus 1d (skip 1d.1 and 1d.2) suggested textbook problems: 1d.1 2, 9,11 26 objective: • know the 4 quantum numbers for atomic orbitals: the allowed values and what it represents • know the shape of the atomic orbitals • know.

Quantum Numbers Pdf Atomic Orbital Applied And Interdisciplinary
