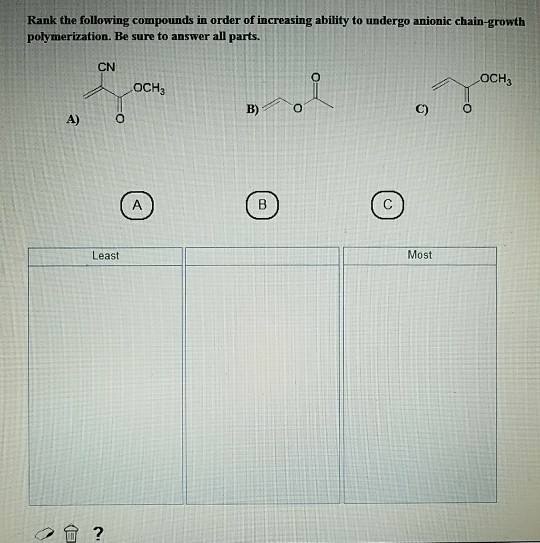
Chemistry Rank The Following Compounds In Order Of Increasing Ability To Undergo Anionic Chain Gr

Solved Rank The Following Compounds In Order Of Increasing Ability To Rank the following compounds in order of increasing ability to undergo anionic chain growth polymerization. be sure to answer all parts. cn och3 och3 c) o a) least most 2. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. Shown below is the order of increasing ability to undergo anionic chain growth polymerization. find step by step solutions and answers to exercise 44 from organic chemistry 9781259629839, as well as thousands of textbooks so you can move forward with confidence.
Solved Rank The Following Compounds In Order Of Increasing Chegg Rank the following compounds in order of increasing ability to undergo anionic chain growth polymerization. The order of the given monomers towards anionic polymerization is as follows: step 1: h2c=ch ch3 step 2: ch2=c (ch3)2 step 3: h2c=ch cooch3 step 4: h2c=ch ch=ch2 thus, the correct order of increasing ability to undergo anionic chain growth polymerization is: h2c=ch ch3 < ch2=c (ch3)2 < h2c=ch cooch3 < h2c=ch ch=ch2. The functional groups present on the monomers and their steric effects are responsible for polymerization through a sequence of reactions that vary in complexity. there exists a stable covalent chemical bond between monomers that sets apart polymerization from other processes. The order of increasing strength of intermolecular forces for the given compounds is isobutane < butane < propylamine, as propylamine can form hydrogen bonds, while the others only exhibit london dispersion forces, with butane having stronger forces than isobutane due to its larger surface area.
Solved Rank The Following Compounds In Order Of Increasing Ability To The functional groups present on the monomers and their steric effects are responsible for polymerization through a sequence of reactions that vary in complexity. there exists a stable covalent chemical bond between monomers that sets apart polymerization from other processes. The order of increasing strength of intermolecular forces for the given compounds is isobutane < butane < propylamine, as propylamine can form hydrogen bonds, while the others only exhibit london dispersion forces, with butane having stronger forces than isobutane due to its larger surface area. Rank the following monomers in order of increasing reactivity toward anionic polymerization (least reactive to most reactive). The electron donating groups on styrene make it undergo cationic polymerization. the electron withdrawing groups on styrene make it undergo anionic polymerization. Step 1 anionic addition polymerization: the polymerization of monomers that is started by anions is known as. Rank the following compounds in order of increasing ability to undergo anionic chain growth polymerization. o o o och3 och3 o cn unfortunately, we don't have that question answered yet. but you can get it answered in just 5 hours by logging in or becoming a subscriber. becoming a subscriber or look for another answer.
Solved Rank The Following Compounds In Order Of Increasing Chegg Rank the following monomers in order of increasing reactivity toward anionic polymerization (least reactive to most reactive). The electron donating groups on styrene make it undergo cationic polymerization. the electron withdrawing groups on styrene make it undergo anionic polymerization. Step 1 anionic addition polymerization: the polymerization of monomers that is started by anions is known as. Rank the following compounds in order of increasing ability to undergo anionic chain growth polymerization. o o o och3 och3 o cn unfortunately, we don't have that question answered yet. but you can get it answered in just 5 hours by logging in or becoming a subscriber. becoming a subscriber or look for another answer.
Comments are closed.