Chemistry Write Balanced Equations For Each Of The Following By Inserting The Correct Coefficients

Solved Write Balanced Equations For Each Of The Following By Inserting Coefficients are the integers placed in front of the formulae that identify the reactants and products. rules associated with formula writing are used to write the reactant and product formulae. when balancing a chemical equation, it is important that the formulae not be changed. Identify the whole number coefficients required to balance the chemical equation so2 (g) o2 (g) >so3 (g) correctly. ( enter all numbers, including the digit 1 if required, and make sure you have the simplest ratio of coefficients.).
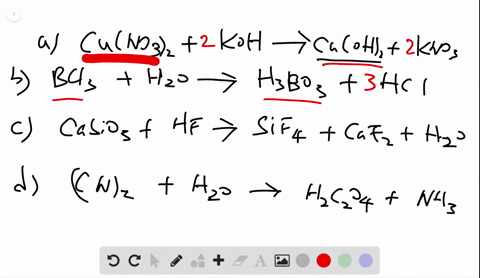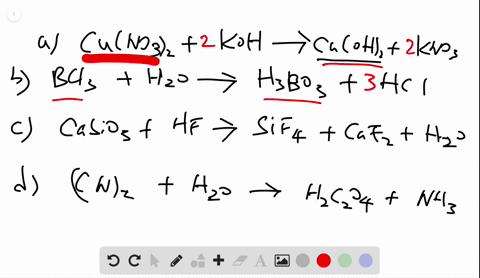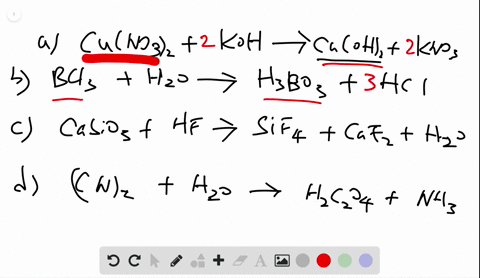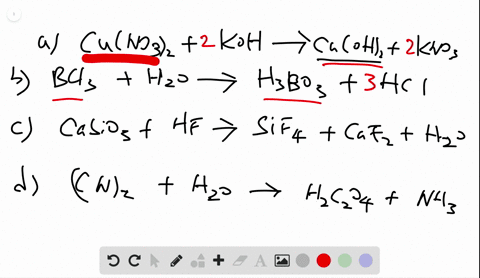
Solved Write Balanced Equations For Each Of The Following By Inserting Balanced chemical equations have the same number and type of each atom on both sides of the equation. the coefficients in a balanced equation must be the simplest whole number ratio. Enter an equation of a chemical reaction and click 'balance'. the answer will appear below. always use the upper case for the first character in the element name and the lower case for the second character. examples: fe, au, co, br, c, o, n, f. compare: co cobalt and co carbon monoxide. Balancing chemical equations is a fundamental principle in chemistry, illustrated in various educational resources and textbooks that emphasize the importance of equal atom counts on both sides. How to balance chemical equations, chemistry balancing tips, law of conservation of mass, balancing equations for beginners.

Solved Write Balanced Equations For Each Of The Following By Chegg Balancing chemical equations is a fundamental principle in chemistry, illustrated in various educational resources and textbooks that emphasize the importance of equal atom counts on both sides. How to balance chemical equations, chemistry balancing tips, law of conservation of mass, balancing equations for beginners. Write and balance chemical equations in molecular, total ionic, and net ionic formats. the preceding chapter introduced the use of element symbols to represent individual atoms. The most common method for balancing equations involves these general steps: step 1: write the correct chemical formulas for all reactants and products. step 2: count the number of atoms of each element on both sides of the arrow. step 3: change the coefficients (the numbers in front of the formulas) to balance the atoms. start with elements that appear in only one reactant and one product. To achieve balance, the coefficients of the equation may be changed as needed. keep in mind, of course, that the formula subscripts define, in part, the identity of the substance, and so these cannot be changed without altering the qualitative meaning of the equation. Write and balance chemical equations in molecular, total ionic, and net ionic formats. the preceding chapter introduced the use of element symbols to represent individual atoms.

Solved Write Balanced Equations For Each Of The Following By Chegg Write and balance chemical equations in molecular, total ionic, and net ionic formats. the preceding chapter introduced the use of element symbols to represent individual atoms. The most common method for balancing equations involves these general steps: step 1: write the correct chemical formulas for all reactants and products. step 2: count the number of atoms of each element on both sides of the arrow. step 3: change the coefficients (the numbers in front of the formulas) to balance the atoms. start with elements that appear in only one reactant and one product. To achieve balance, the coefficients of the equation may be changed as needed. keep in mind, of course, that the formula subscripts define, in part, the identity of the substance, and so these cannot be changed without altering the qualitative meaning of the equation. Write and balance chemical equations in molecular, total ionic, and net ionic formats. the preceding chapter introduced the use of element symbols to represent individual atoms.

Solved Write Balanced Equations For Each Of The Following By Chegg To achieve balance, the coefficients of the equation may be changed as needed. keep in mind, of course, that the formula subscripts define, in part, the identity of the substance, and so these cannot be changed without altering the qualitative meaning of the equation. Write and balance chemical equations in molecular, total ionic, and net ionic formats. the preceding chapter introduced the use of element symbols to represent individual atoms.
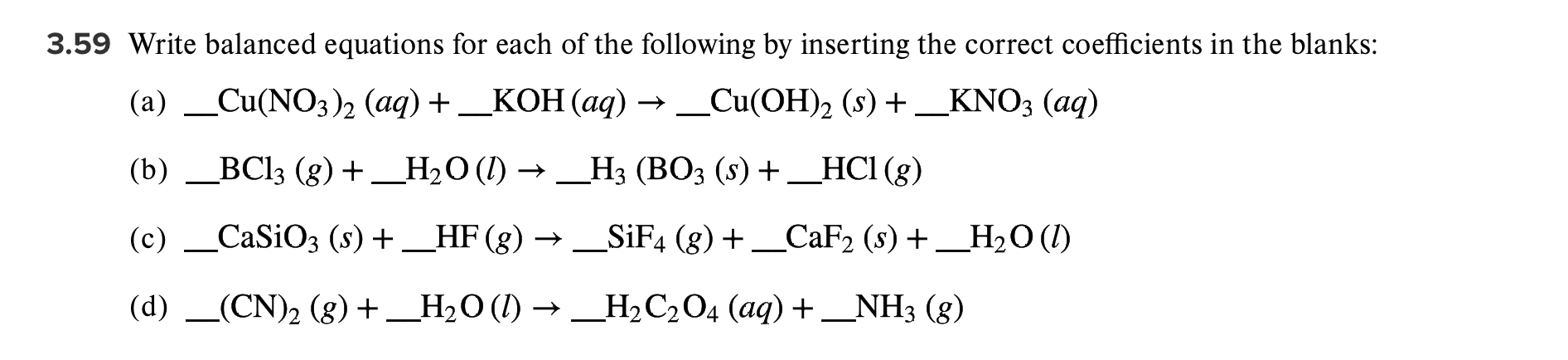
Solved 3 59 Write Balanced Equations For Each Of The Chegg
Comments are closed.