Consider The Diagram Of An Aqueous Solution Of A Chegg
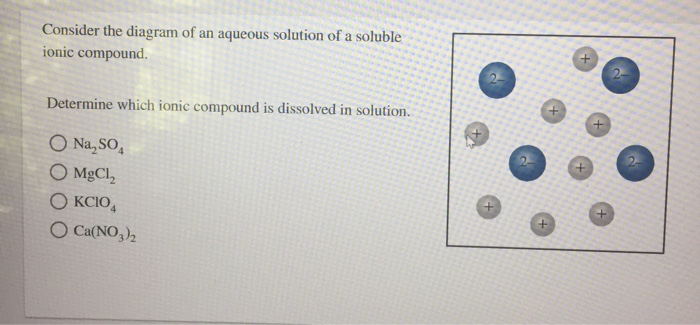
Solved Consider The Diagram Of An Aqueous Solution Of A Chegg Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. see answer. To determine which ionic compound is dissolved in the aqueous solution, we need to consider how each compound dissociates in water and match it with the description provided by the diagram, which is not visible here but we will use typical dissociation patterns.

Solved Consider The Diagram Of An Aqueous Solution Of A Chegg To determine which ionic compound is dissolved in the solution based on the diagram, we need to understand the nature of the ions present in each compound and their behavior when dissolved in water. Consider the diagram of an aqueous solution of a soluble ionic compound. determine which ionic compound is dissolved in solution. kci mgcro4 ca (cio4)2 cabr2. ionic compounds are formed through the electrostatic attraction between oppositely charged ions. they dissociate into their constituent ions when dissolved in water. Each image depicts what happens when different types of compounds are dissolved in aqueous solution. classify each image as that of a strong electrolyte, weak electrolyte, or non electrolyte. Use the solubility rules to predict whether the following compounds are soluble or insoluble in water. if a compound is soluble, write the net ionic equation for the dissolution of the compound into its component ions in water. a. nac n a c l. b. pbc2 p b c l 2. d. nh4no3 n h 4 n o 3. e. kbr k b r.
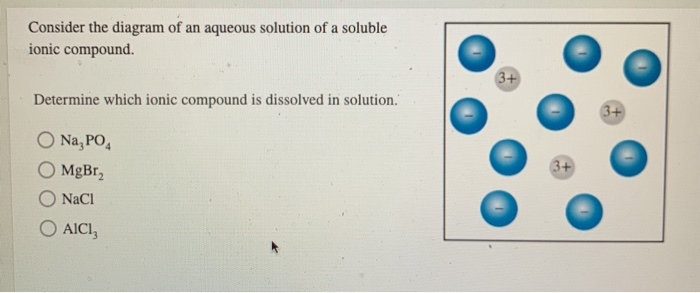
Solved Consider The Diagram Of An Aqueous Solution Of A Chegg Each image depicts what happens when different types of compounds are dissolved in aqueous solution. classify each image as that of a strong electrolyte, weak electrolyte, or non electrolyte. Use the solubility rules to predict whether the following compounds are soluble or insoluble in water. if a compound is soluble, write the net ionic equation for the dissolution of the compound into its component ions in water. a. nac n a c l. b. pbc2 p b c l 2. d. nh4no3 n h 4 n o 3. e. kbr k b r. In the diagrams, if a and b are no longer bonded together, you should consider the separated a component as having a 1 charge and separated b as having a 1 charge. Consider the energy diagram for a solution formed between molecules a and b. what can be said about this solution? a) forming the solution is exothermic. b) the solute solvent attractions are weak compared to the solute solute and solvent solvent attractions. c) the solute solute attractions are much stronger than the solvent solvent attractions. To determine which ionic compound is dissolved in the solution, we would typically analyze the properties and composition of the solution. however, since the provided information is limited to just a list of possible compounds, it's not possible to determine the exact compound dissolved in solution without additional details such as:. For an aqueous solution, this diagram depicts the coexistence regions of solid, liquid, and gaseous phases, alongside their transitions. the key feature is the identification of saturation points, beyond which further solute cannot dissolve, leading to precipitation or crystallization.
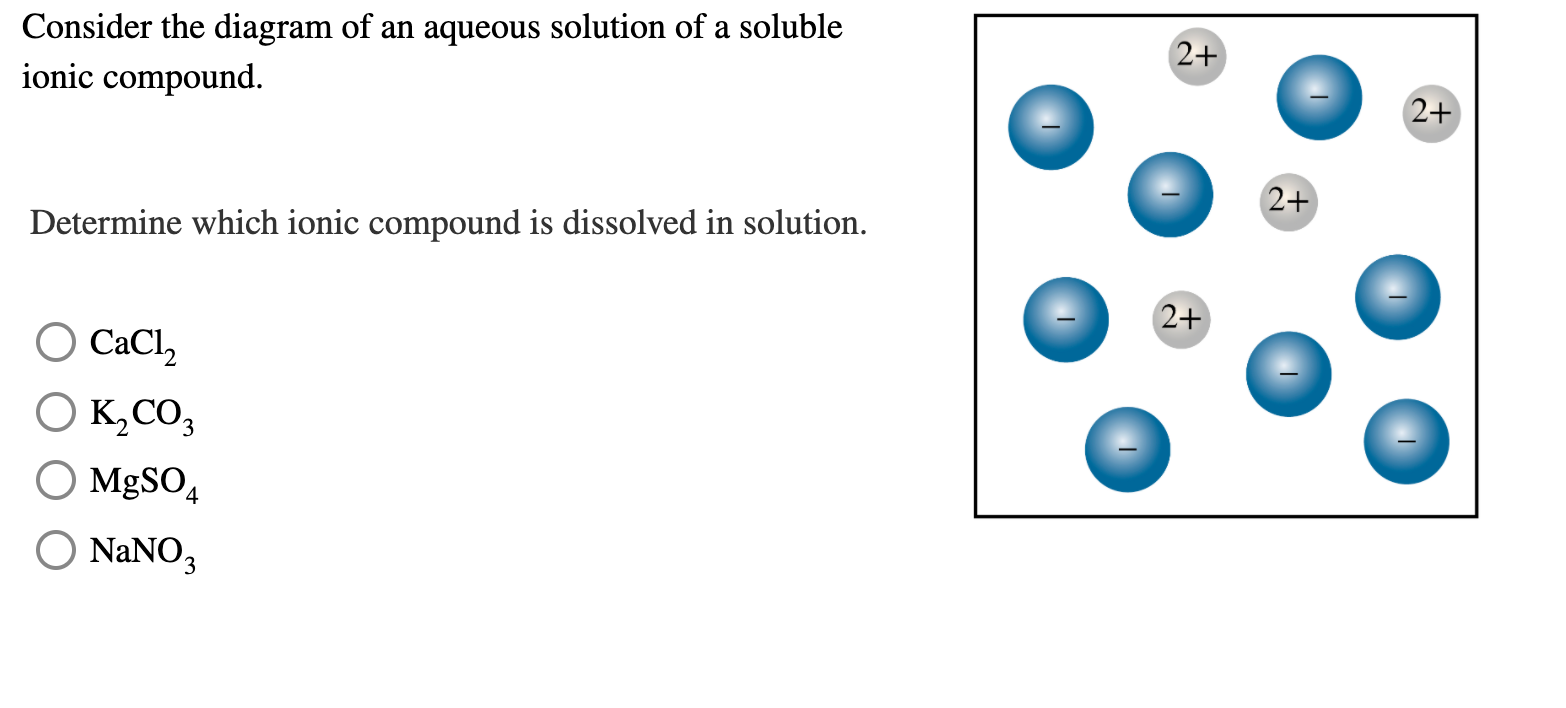
Solved Consider The Diagram Of An Aqueous Solution Of A Chegg In the diagrams, if a and b are no longer bonded together, you should consider the separated a component as having a 1 charge and separated b as having a 1 charge. Consider the energy diagram for a solution formed between molecules a and b. what can be said about this solution? a) forming the solution is exothermic. b) the solute solvent attractions are weak compared to the solute solute and solvent solvent attractions. c) the solute solute attractions are much stronger than the solvent solvent attractions. To determine which ionic compound is dissolved in the solution, we would typically analyze the properties and composition of the solution. however, since the provided information is limited to just a list of possible compounds, it's not possible to determine the exact compound dissolved in solution without additional details such as:. For an aqueous solution, this diagram depicts the coexistence regions of solid, liquid, and gaseous phases, alongside their transitions. the key feature is the identification of saturation points, beyond which further solute cannot dissolve, leading to precipitation or crystallization.
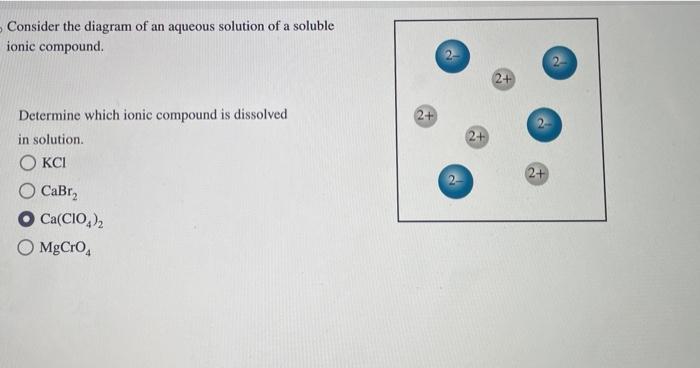
Solved Consider The Diagram Of An Aqueous Solution Of A Chegg To determine which ionic compound is dissolved in the solution, we would typically analyze the properties and composition of the solution. however, since the provided information is limited to just a list of possible compounds, it's not possible to determine the exact compound dissolved in solution without additional details such as:. For an aqueous solution, this diagram depicts the coexistence regions of solid, liquid, and gaseous phases, alongside their transitions. the key feature is the identification of saturation points, beyond which further solute cannot dissolve, leading to precipitation or crystallization.
Solved Consider The Diagram Of An Aqueous Solution Of A Chegg
Comments are closed.