Consider The Following Compoundsko2 H2o2 And H2so4 The Oxidation States Of The Underlined Element

Hc тйб Ch Hg 2 Dil H2so4 Ch3ch O Which Statement S Is Are Correct option is: (1) 1, –1, and 6. \ (ko 2 \rightarrow \) alkali metal always shows 1 oxidation state. therefore oxidation state of k is 1. \ (h 2o 2 \rightarrow\) oxidation state of oxygen in \ (h 2 o 2\) is –1. \ (h 2 so 4\rightarrow\) oxidation state of sulphur in \ (h 2 so 4\) is 6. In these compounds, the underlined elements are chosen so that we use common oxidation state rules: in ko₂ the underlined element is k, which always has an oxidation state of 1.

Solved Balance The Following Equation K2so4 2koh â K2so3 H2o Thus, the oxidation states of the elements in h 2 2 o 2 2 are h = 1 and o = 1. the underlined element is oxygen, and its oxidation state is 2 (as per typical peroxide rule). Therefore, sulfur (s) has an oxidation state of 6. therefore, the oxidation states of the underlined elements in ko₂, h₂o₂, and h₂so₄ are respectively 1, 1, and 6. Q.57) consider the following compounds : $\mathrm {ko} 2, \mathrm {h} 2 \mathrm {o} 2$ and $\mathrm {h} 2 \mathrm {so} 4$. the oxidation states of the underlined elements in them are, respectively: a) $ 4, 4$, and 6 b) $ 1, 1$, and 6 c) $ 2, 2$, and 6 d) $ 1, 2$, and 4. Subtopic: introduction to redox and oxidation number | 65% from ncert neet 2022.
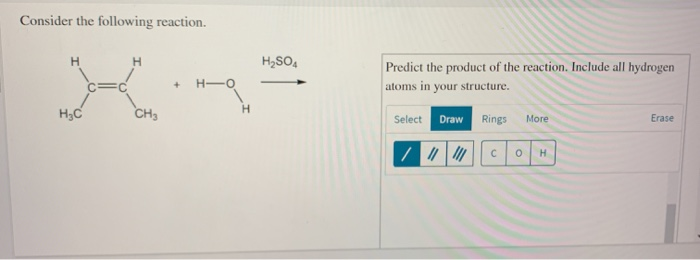
Solved Consider The Following Reaction H2so4 Ho C Chegg Q.57) consider the following compounds : $\mathrm {ko} 2, \mathrm {h} 2 \mathrm {o} 2$ and $\mathrm {h} 2 \mathrm {so} 4$. the oxidation states of the underlined elements in them are, respectively: a) $ 4, 4$, and 6 b) $ 1, 1$, and 6 c) $ 2, 2$, and 6 d) $ 1, 2$, and 4. Subtopic: introduction to redox and oxidation number | 65% from ncert neet 2022. Neet 2025 pyq chemistry consider the following compounds:ko2,h2o2 and h2so4.the oxidation states of the underlined elements in them are, respectively, (1) 1,. To determine the oxidation states of the underlined elements in the compounds k2o2, h2o2, and h2so4, we will analyze each compound separately. for k2 o2 (potassium peroxide):. Oxygen in superoxide ion ($\ce {o2^ }$) has an oxidation state of 1. for $\ce {h2o2}$, each oxygen has an oxidation state of 1. for $\ce {h2so4}$, sulfur has an oxidation state of 6. 2. thus, the correct oxidation states are 1 for k, 1 for o in $\ce {h2o2}$, and 6 for s in $\ce {h2so4}$. To calculate the oxidation numbers of the underlined elements in the given compounds, we will follow a systematic approach for each compound. (i) for cao2 (calcium oxide):.
Comments are closed.