Consider The Following Compoundsko2h2o2 And H2so4 The Oxidation States Of The Underlined Elements

Solved Balance The Following Equation K2so4 2koh â K2so3 H2o Correct option is: (1) 1, –1, and 6. \ (ko 2 \rightarrow \) alkali metal always shows 1 oxidation state. therefore oxidation state of k is 1. \ (h 2o 2 \rightarrow\) oxidation state of oxygen in \ (h 2 o 2\) is –1. \ (h 2 so 4\rightarrow\) oxidation state of sulphur in \ (h 2 so 4\) is 6. In these compounds, the underlined elements are chosen so that we use common oxidation state rules: in ko₂ the underlined element is k, which always has an oxidation state of 1.

Hc тйб Ch Hg 2 Dil H2so4 Ch3ch O Which Statement S Is Are Thus, the oxidation states of the elements in h 2 2 o 2 2 are h = 1 and o = 1. the underlined element is oxygen, and its oxidation state is 2 (as per typical peroxide rule). Consider the following compounds:ko2, h2o2 and h2so4 the oxidation states of the underlined elements in them are, respectively, (1) 1, –1, and 6 (2) 2, –2,. Therefore, sulfur (s) has an oxidation state of 6. therefore, the oxidation states of the underlined elements in ko₂, h₂o₂, and h₂so₄ are respectively 1, 1, and 6. In sulfuric acid (h2so4), the oxidation state of sulfur is determined by solving the equation for a neutral molecule: 2 ( 1) s 4 (–2) = 0 solving gives: 2 s – 8 = 0 ⇒ s = 6 so, sulfur has an oxidation state of 6 in h2so4.
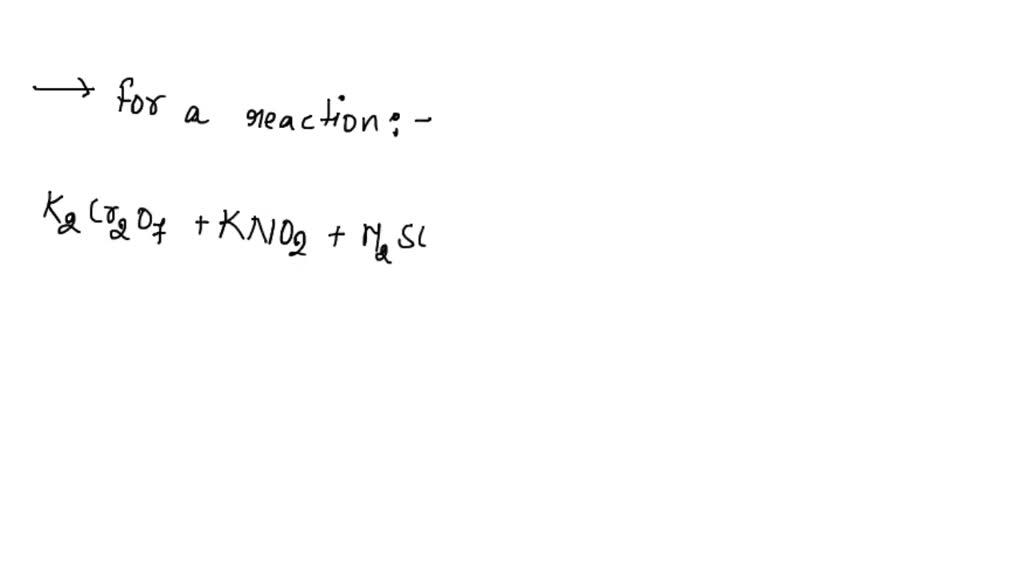
Solved K2cr2o7 Kno2 H2so4 Cr2 So4 3 Kno3 K2so4 H2o 1 Therefore, sulfur (s) has an oxidation state of 6. therefore, the oxidation states of the underlined elements in ko₂, h₂o₂, and h₂so₄ are respectively 1, 1, and 6. In sulfuric acid (h2so4), the oxidation state of sulfur is determined by solving the equation for a neutral molecule: 2 ( 1) s 4 (–2) = 0 solving gives: 2 s – 8 = 0 ⇒ s = 6 so, sulfur has an oxidation state of 6 in h2so4. Subtopic: introduction to redox and oxidation number | 81% from ncert neet 2024. Q.57) consider the following compounds : $\mathrm {ko} 2, \mathrm {h} 2 \mathrm {o} 2$ and $\mathrm {h} 2 \mathrm {so} 4$. the oxidation states of the underlined elements in them are, respectively: a) $ 4, 4$, and 6 b) $ 1, 1$, and 6 c) $ 2, 2$, and 6 d) $ 1, 2$, and 4. Let's find the oxidation states of the underlined elements in the compounds given. potassium (k) has an oxidation state of 1. the compound contains the superoxide ion o₂⁻. Neet 2025 pyq chemistry consider the following compounds:ko2,h2o2 and h2so4.the oxidation states of the underlined elements in them are, respectively, (1) 1,.
Comments are closed.