Consider The Following Equilibrium Cog 2h2g Ch3ohg 0 1 Mol Of Co Along With A Catalyst Is

Solved Question 1 Consider The Following Equilibrium Not Chegg 0.1 mol of co along with a catalyst is present in a \ (2 \ dm^3\) flast maintained at 500 k. hydrogen is introduced into the flask until the pressure is 5 bar and 0.04 mol of \ (ch 3oh\) is formed. The equilibrium constant expression for the reaction co (g) 2h2 (g) ⇌ ch3oh (g) is given by k eq = [ch3oh] ( [co] [h2]^2). therefore, the correct option is b.

Solved Consider The Following Equilibrium H2o G Cog 2 Chegg Consider the following reaction: co (g) 2h2 (g)⇌ch3oh (g) an equilibrium mixture of this reaction at a certain temperature was found to have [co]= 0.105 m , [h2]= 0.114 m , and [ch3oh]= 0.180 m . For the reaction: ch 3 oh (g) ↔ co (g) 2h 2 (g), with the equilibrium concentrations for [ch 3 oh]=0.20m, [co]=0.44m, and [h 2]=2.7m, determine the gas phase equilibrium constant (k p) at 400 0 c?. An ice (initial change equilibrium) table is useful here to systematically determine the change in moles of each species as the reaction proceeds. This value indicates the ratio of product concentration to reactant concentrations at equilibrium for this reaction at the given temperature. a kc value greater than 1 suggests that, at equilibrium, the reaction favors the formation of products under the conditions specified.
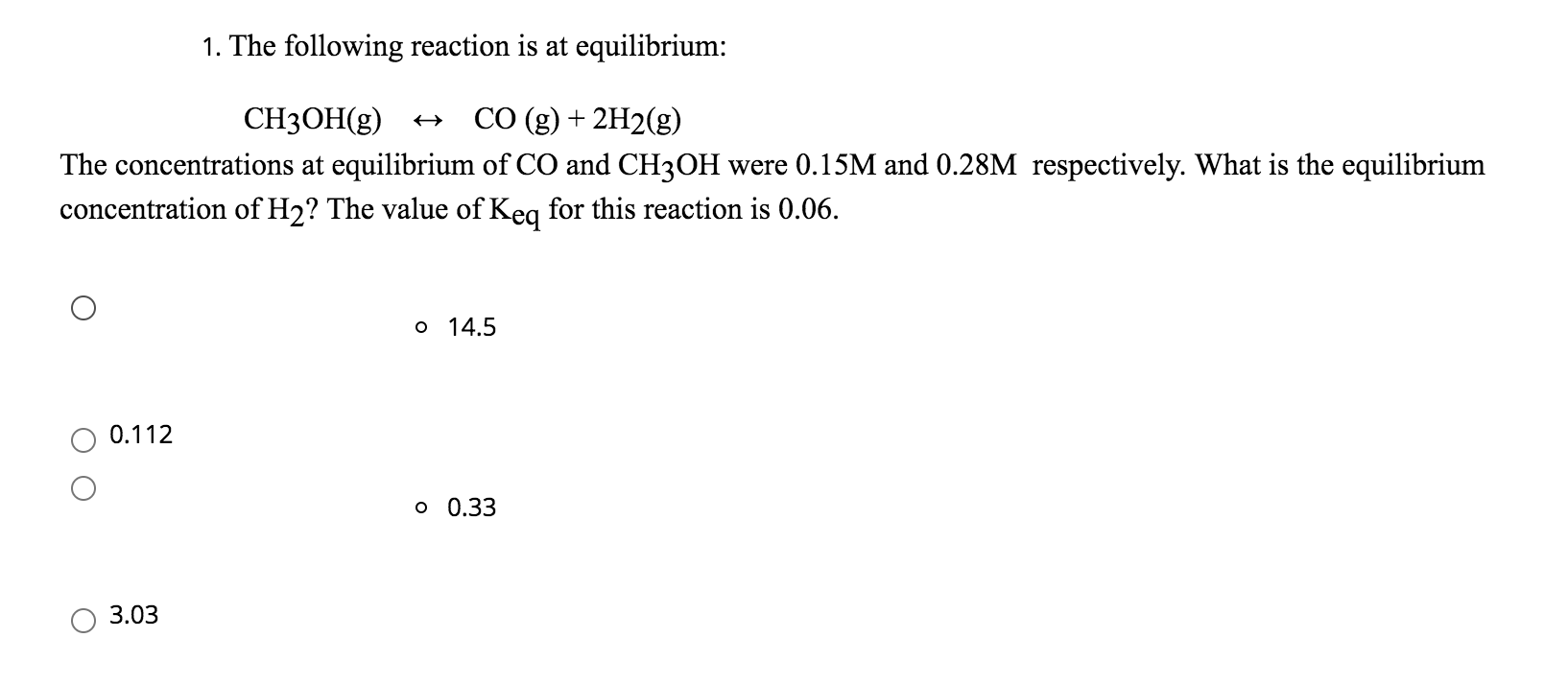
Solved 1 The Following Reaction Is At Equilibrium Ch3oh G Chegg An ice (initial change equilibrium) table is useful here to systematically determine the change in moles of each species as the reaction proceeds. This value indicates the ratio of product concentration to reactant concentrations at equilibrium for this reaction at the given temperature. a kc value greater than 1 suggests that, at equilibrium, the reaction favors the formation of products under the conditions specified. By comparing q to kp, one can determine the direction in which the reaction will proceed to reach equilibrium: if q < kp, the reaction will shift to the right (toward products), and if q > kp, it will shift to the left (toward reactants). What is the value of the equilibrium constant (kc) at this temperature? answer with three sig figs. solution verified answered 1 year ago. This isn't provided for the ch3oh (g) ⇌ co (g) 2h2 (g) reaction directly. instead, we can use the van't hoff equation for reactions at equilibrium, which doesn't require Δh° and Δs° but does need the equilibrium constant (kp). Consider the reaction: co (g) 2 h2 (g) ⇌ ch3oh (g). an equilibrium mixture of this reaction at a certain temperature has [co] = 0.105 m, [h2] = 0.114 m, and [ch3oh] = 0.185 m.
Comments are closed.