Consider The Following Unbalanced Equation Pbno32aq Naclaq Pbcl2s Nano3aq If 4

Solved Consider The Following Unbalanced Equation Which Of Chegg Question: consider the following unbalanced equation:pb (no3)2 (aq) nacl (aq) > pbcl2 (s) nano3 (aq). if 455 ml of a 0.376 m solution of nacl is added to excess pb (no3)2, how many individual compounds of pbcl2 will be produced?. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. a balanced equation obeys the law of conservation of mass, which states that matter is neither created nor destroyed in a chemical reaction.
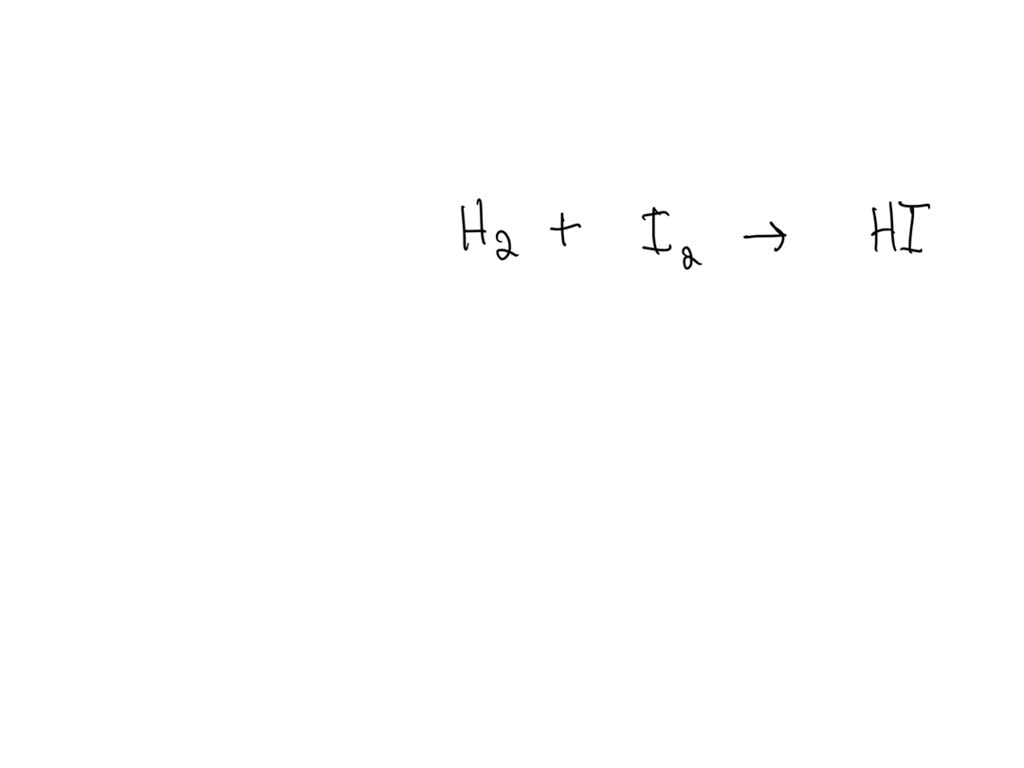
Solved Consider The Following Unbalanced Particulate Representation Of To balance the given chemical equation, a coefficient of 2 should be placed in front of nacl. this balances the number of chlorine atoms on both sides of the equation, resulting in a balanced reaction. Consider the following unbalanced equation: pb (no3)2 (aq) nacl (aq) — gt; pbcl2 (s) nano3 (aq). if 455 ml of a 0.376 m solution of nacl is added to excess. In the following reaction, when the equation is correctly balanced, what is the correct coefficient for lead (ii) chloride? ? pb (no3)2 (aq) ? nacl (aq) ——> ? pbcl2 (s) ? nano3 (aq). Consider the following unbalanced equation: pb(no3)2(aq) nacl(aq) > pbcl2(s) nano3(aq). if 455 ml of a 0.376 m solution of nacl is added to excess pb(no3)2, how many individual compounds of pbcl2 will be produced? please show the answer in detail.
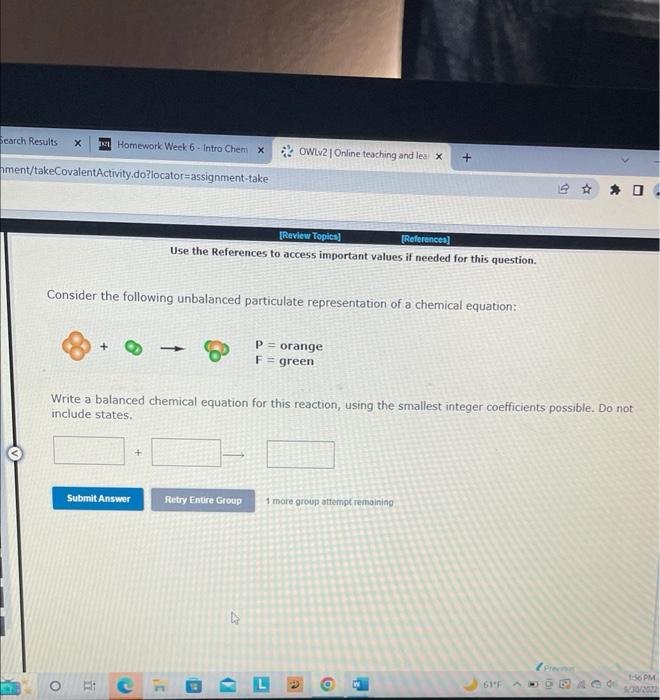
Solved Consider The Following Unbalanced Particulate Chegg In the following reaction, when the equation is correctly balanced, what is the correct coefficient for lead (ii) chloride? ? pb (no3)2 (aq) ? nacl (aq) ——> ? pbcl2 (s) ? nano3 (aq). Consider the following unbalanced equation: pb(no3)2(aq) nacl(aq) > pbcl2(s) nano3(aq). if 455 ml of a 0.376 m solution of nacl is added to excess pb(no3)2, how many individual compounds of pbcl2 will be produced? please show the answer in detail. Since there are an equal number of atoms of each element on both sides, the equation is balanced. Consider the following equation: [5 marks] 2 nacl (aq) pb (no3)2 (aq) → 2 nano3 (aq) pbcl2 (s) a. how many grams of lead (ii) chloride are produced from the reaction of 10.3 g of nacl and 50.8 g of pb (no3)2?. In the following reaction, when the equation is correctly balanced, what is the correct coefficient for sodium chloride? pb (no3)2 (aq) nacl (aq) → pbcl2 (s) nano3 (aq). In this video we'll balance the equation pb (no3)2 nacl = pbcl2 nano3 and provide the correct coefficients for each compound. more.

Solved Question 3 1 Pts Consider The Following Unbalanced Chegg Since there are an equal number of atoms of each element on both sides, the equation is balanced. Consider the following equation: [5 marks] 2 nacl (aq) pb (no3)2 (aq) → 2 nano3 (aq) pbcl2 (s) a. how many grams of lead (ii) chloride are produced from the reaction of 10.3 g of nacl and 50.8 g of pb (no3)2?. In the following reaction, when the equation is correctly balanced, what is the correct coefficient for sodium chloride? pb (no3)2 (aq) nacl (aq) → pbcl2 (s) nano3 (aq). In this video we'll balance the equation pb (no3)2 nacl = pbcl2 nano3 and provide the correct coefficients for each compound. more.

Solved References Consider The Following Unbalanced Chegg In the following reaction, when the equation is correctly balanced, what is the correct coefficient for sodium chloride? pb (no3)2 (aq) nacl (aq) → pbcl2 (s) nano3 (aq). In this video we'll balance the equation pb (no3)2 nacl = pbcl2 nano3 and provide the correct coefficients for each compound. more.

Solved Consider The Following Unbalanced Chegg
Comments are closed.