Difference Between Acids And Bases Key Properties Yourdictionary
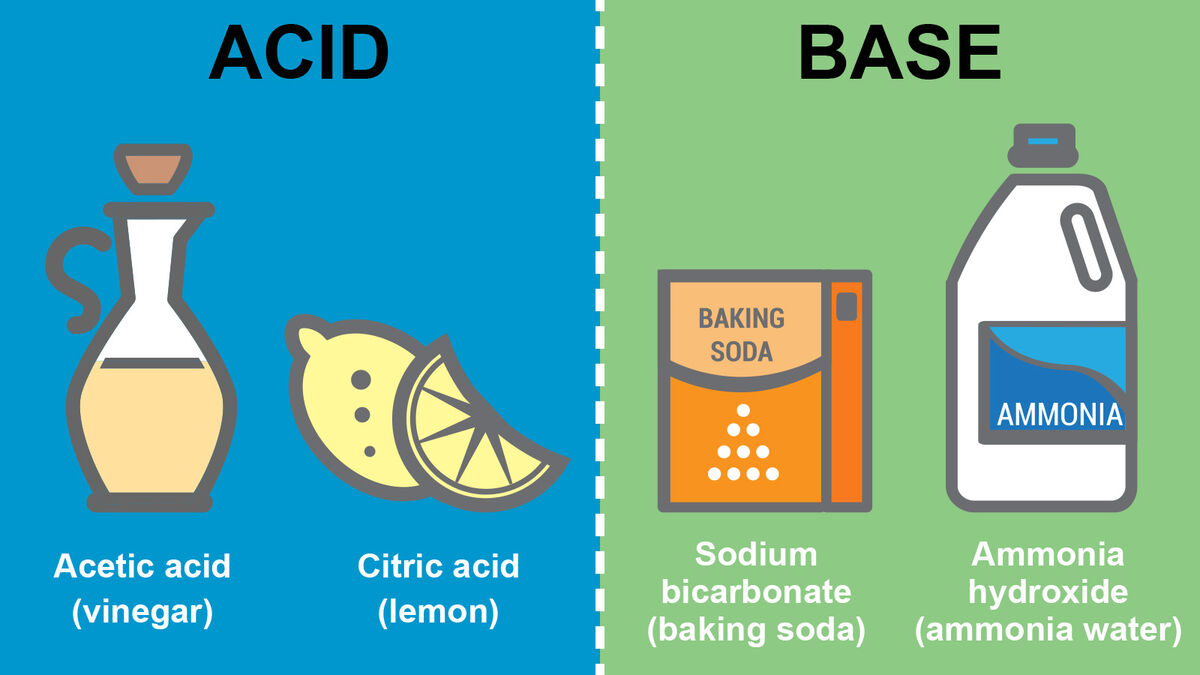
Difference Between Acids And Bases Key Properties Yourdictionary Bases are the chemical opposite of acids. acids are defined as compounds that donate a hydrogen ion (h ) to another compound (called a base). Discover the physical and chemical properties of acids and bases. learn the key differences between acids and bases and explore the common examples in everyday life.
Acids And Bases Learn the key differences between acids and bases with clear tables, daily life examples, and exam focused tips. ideal for quick revision and concept clarity. Acids, in their most primal form, are proton givers. they donate hydrogen ions, tiny positively charged packets of energy, to other molecules or ions. bases, their eternal dance partners, are proton takers — either by snatching up hydrogen ions or by donating electrons that attract these protons. Learn how acids and bases fundamentally differ. explore their unique characteristics and vital roles in chemistry and everyday life. Acids and bases react with a wide range of chemical compounds to form salts. the physical properties of acids and bases are listed in the table below. mineral acids are colourless liquids but sometimes sulphuric acid becomes yellow due to impurities. some organic acids are white coloured solids.

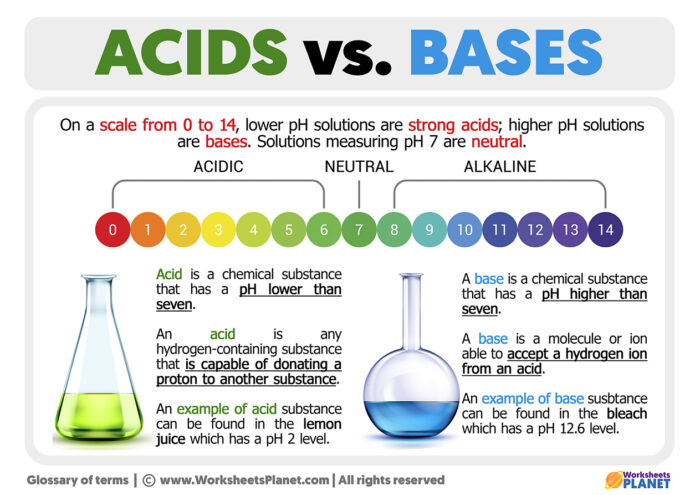
Difference Between Acids And Bases Learn how acids and bases fundamentally differ. explore their unique characteristics and vital roles in chemistry and everyday life. Acids and bases react with a wide range of chemical compounds to form salts. the physical properties of acids and bases are listed in the table below. mineral acids are colourless liquids but sometimes sulphuric acid becomes yellow due to impurities. some organic acids are white coloured solids. Here are some of the key differences between acids and bases: 1. definition: according to arrhenius theory, an acid is a substance that produces hydrogen ions (h ) when dissolved in water, while a base is a substance that produces hydroxide ions (oh ) when dissolved in water. Acids vs bases. master the difference with our complete guide. learn ph scale, properties, examples, and safety tips. Learn the difference between acids and bases, their properties, ph levels, and real life uses. understand acid base reactions with this complete chemistry guide. A ph value below 7 indicates an acidic solution, with values closer to 0 representing stronger acids. conversely, a ph value above 7 signifies a basic, or alkaline, solution, where values nearer to 14 denote stronger bases. a ph value of exactly 7 indicates a neutral solution, such as pure water.
Comments are closed.