Fda Requirements For Medical Devices Prioritize Cybersecurity To Avoid

Fda Requirements For Medical Devices Prioritize Cybersecurity To Avoid The fda is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and by ensuring the. The fda, an agency within the u.s. department of health and human services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines.

Fda Class Ii Medical Devices 510 K Compliance Checklist Recalls, market withdrawals, & safety alerts the list below provides information gathered from press releases and other public notices about certain recalls of fda regulated products. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. The u.s. food and drug administration today is taking a bold step to protect americans from dangerous, illegal opioids by recommending a scheduling action to control certain 7 hydroxymitragynine. On oct. 1, 2024, the fda began implementing a reorganization impacting many parts of the agency. we are in the process of updating fda.gov content to reflect these changes.

Fda Protects Medical Devices Against Cyber Threats With New Measures The u.s. food and drug administration today is taking a bold step to protect americans from dangerous, illegal opioids by recommending a scheduling action to control certain 7 hydroxymitragynine. On oct. 1, 2024, the fda began implementing a reorganization impacting many parts of the agency. we are in the process of updating fda.gov content to reflect these changes. The food and drug administration is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical. Novel drugs at fda: cder’s new molecular entities and new therapeutic biological products drug and biologic approval and ind activity reports this week's drug approvals drug trials snapshots. By phone: call 1 888 info fda (1 888 463 6332). call the fda consumer complaint coordinator for your state or region. for more details, see how to report a problem. Página principal en español de la administración de alimentos y medicamentos de los estados unidos (fda).

Fda Medical Device Recalls Classes Process Compliance The food and drug administration is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical. Novel drugs at fda: cder’s new molecular entities and new therapeutic biological products drug and biologic approval and ind activity reports this week's drug approvals drug trials snapshots. By phone: call 1 888 info fda (1 888 463 6332). call the fda consumer complaint coordinator for your state or region. for more details, see how to report a problem. Página principal en español de la administración de alimentos y medicamentos de los estados unidos (fda).
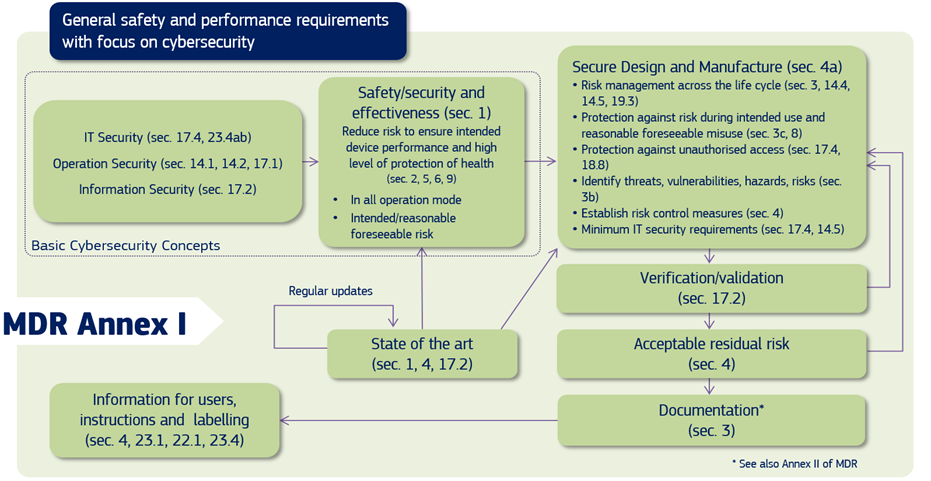
Cybersecurity For Medical Devices Omc Medical By phone: call 1 888 info fda (1 888 463 6332). call the fda consumer complaint coordinator for your state or region. for more details, see how to report a problem. Página principal en español de la administración de alimentos y medicamentos de los estados unidos (fda).
Comments are closed.