For The Reaction2a B A2b The Rate Kab2 With K 2 0 X 10 6 Mol 2 L2 S 1 Calculate

For The Reaction 2a B A2b The Rate K A B 2 With K 2 0 10 For the reaction: 2a b → a2b the rate = k [a] [b]2 with k = 2.0 × 10−6 mol−2 l2 s−1. calculate the initial rate of the reaction when [a] = 0.1 mol l−1, [b] = 0.2 mol l−1. calculate the rate of reaction after [a] is reduced to 0.06 mol l−1. Calculate the initial rate of the reaction when [a] = 0.1m, [b] = 0.2 m. if the rate of reverse reaction is negligible then calculate the rate of reaction after [a] is reduced to 0.06 m.

Solved What Is The Rate Law Rate K A 2 B Rate Chegg To solve for the initial rate of reaction for the equation 2a b → a2b, where the rate is given by the expression rate = k[a][b]2, we are given: let's calculate the initial rate of reaction: now, let's calculate the rate when [a] = 0.06 mol l−1:. If the value of k is 2.0 × 10^ 6 mol^ 2l^2s^ 1 for the reaction, determine the initial rate of the reaction with [a] = 0.2 mol l^ 1, [b] = 0.1 mol l^ 1, [c] = 0.5 mol l^ 1. For the reaction: 2a b → a2b the rate = k [a] [b]2 with k = 2.0 × 10–6 mol–2 l2 s–1. calculate the initial rate of t more. Question for the reaction: 2a b → a2b the rate = k[a][b]2 with k =2.0×10−6mol−2l2s−1. calculate the initial rate of the reaction when [a] =0.1moll−1, [b] = 0.2moll−1. calculate the rate of reaction after [a] is reduced to 0.06moll−1. solution verified by toppr.
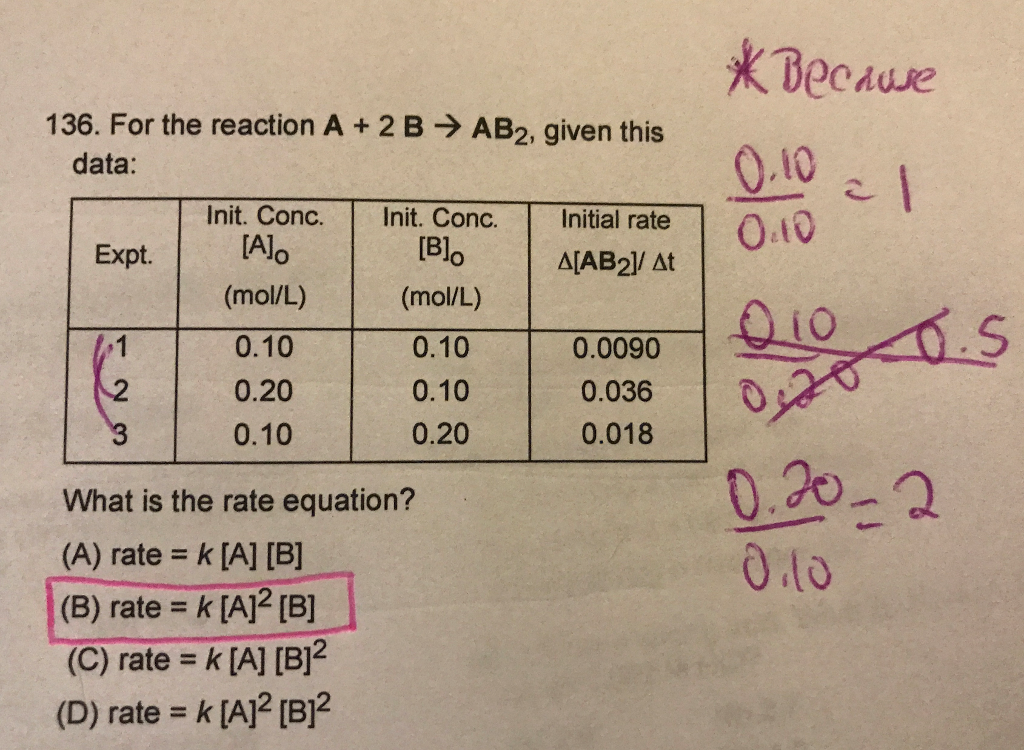
Solved For The Reaction A 2 B Rightarrow Ab 2 Given This Chegg For the reaction: 2a b → a2b the rate = k [a] [b]2 with k = 2.0 × 10–6 mol–2 l2 s–1. calculate the initial rate of t more. Question for the reaction: 2a b → a2b the rate = k[a][b]2 with k =2.0×10−6mol−2l2s−1. calculate the initial rate of the reaction when [a] =0.1moll−1, [b] = 0.2moll−1. calculate the rate of reaction after [a] is reduced to 0.06moll−1. solution verified by toppr. To solve the problem, we will follow these steps: step 1: calculate the initial rate of the reaction. substituting the values into the rate equation: rate =(2.0×10−6)×(0.1)×(0.2)2. calculating (0.2)2 = 0.04: rate =(2.0×10−6)×(0.1)×(0.04) calculating the product: rate =(2.0×10−6)×(0.004) = 8.0×10−9 mol l−1s−1. Calculate the initial rate of the reaction when [a] = 0.1 mol l−1, [b] = 0.2 mol l−1. calculate the rate of reaction after [a] is reduced to 0.06 mol l−1. For the reaction 2a b → a2b, rate = k [a] [b]2 with k = 20 x 10 6 mol 2 l2 s 1. calculate the initial rate of the reaction when [a] = 0.1 mol l 1 and [b] = 0.2 mol l 1 calculate the rate of reaction after [a] is reduced to 0.06 mol l 1. Q. fill in the blanks in the following table which treats reaction of a compound a with a compound b, that is of the first order with respect to a and zero order with respect to b.
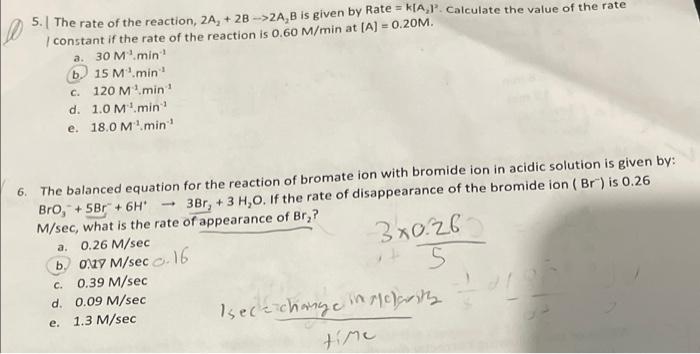
Solved 2 For A Given Reaction 2a B 2 Ab Rate Chegg To solve the problem, we will follow these steps: step 1: calculate the initial rate of the reaction. substituting the values into the rate equation: rate =(2.0×10−6)×(0.1)×(0.2)2. calculating (0.2)2 = 0.04: rate =(2.0×10−6)×(0.1)×(0.04) calculating the product: rate =(2.0×10−6)×(0.004) = 8.0×10−9 mol l−1s−1. Calculate the initial rate of the reaction when [a] = 0.1 mol l−1, [b] = 0.2 mol l−1. calculate the rate of reaction after [a] is reduced to 0.06 mol l−1. For the reaction 2a b → a2b, rate = k [a] [b]2 with k = 20 x 10 6 mol 2 l2 s 1. calculate the initial rate of the reaction when [a] = 0.1 mol l 1 and [b] = 0.2 mol l 1 calculate the rate of reaction after [a] is reduced to 0.06 mol l 1. Q. fill in the blanks in the following table which treats reaction of a compound a with a compound b, that is of the first order with respect to a and zero order with respect to b.
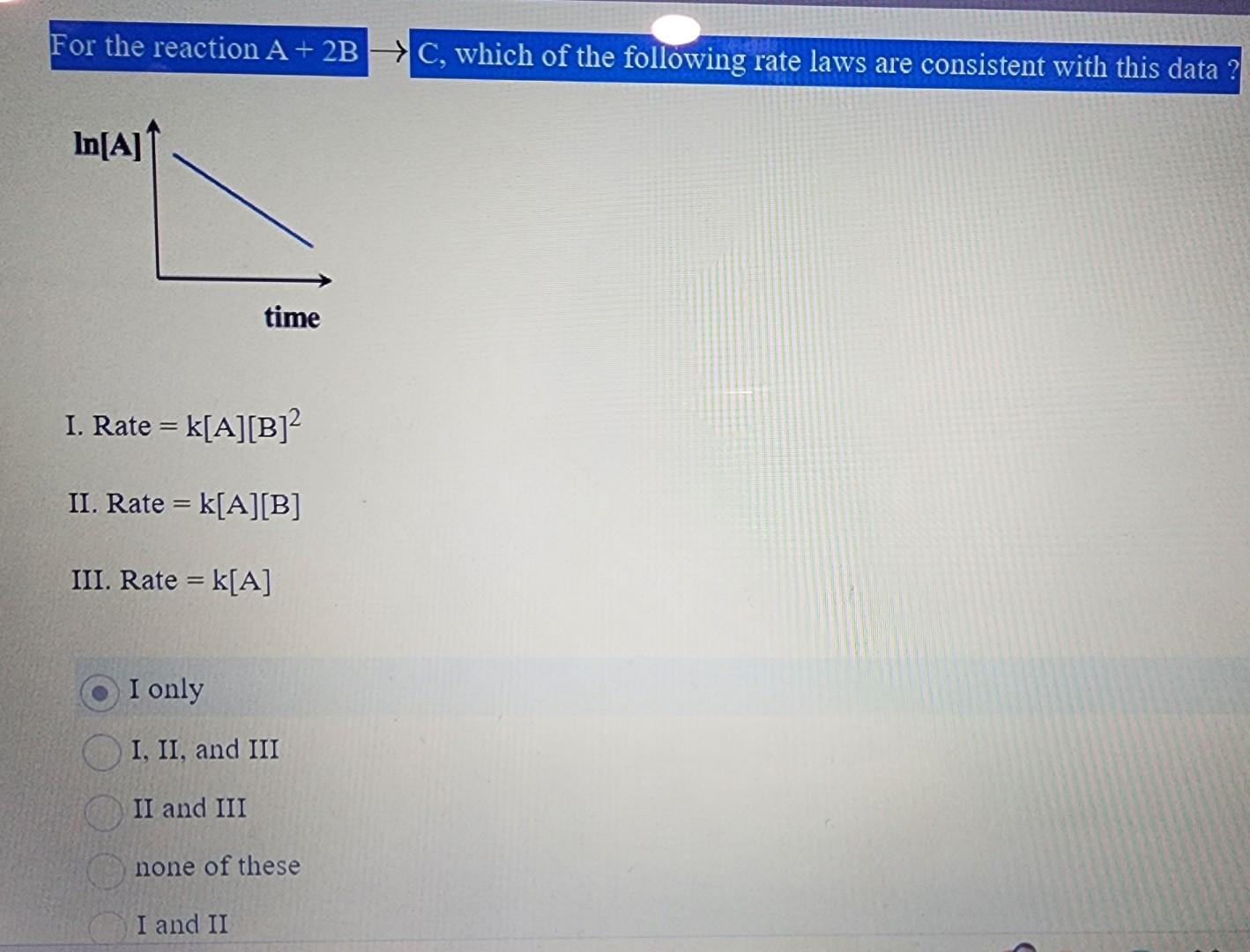
Solved Rate K A B 2 Rate K A B Ii Rate K A I Only I Chegg For the reaction 2a b → a2b, rate = k [a] [b]2 with k = 20 x 10 6 mol 2 l2 s 1. calculate the initial rate of the reaction when [a] = 0.1 mol l 1 and [b] = 0.2 mol l 1 calculate the rate of reaction after [a] is reduced to 0.06 mol l 1. Q. fill in the blanks in the following table which treats reaction of a compound a with a compound b, that is of the first order with respect to a and zero order with respect to b.
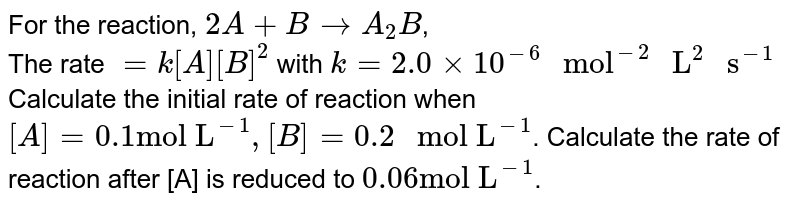
For The Reaction 2a B C A2b C The Rate K A B 2 Wi
Comments are closed.