Half Reaction Method Balancing Redox Reactions In Basic Acidic Solution Chemistry

Half Reaction Method Balancing Redox Reactions In Basic Acidic This chemistry video tutorial provides a basic introduction into the half reaction method which is useful for balancing redox reactions in basic solution and in acidic. Another method for balancing redox reactions uses half reactions. recall that a half reaction is either the oxidation or reduction that occurs, treated separately. the half reaction method works better than the oxidation number method when the substances in the reaction are in aqueous solution.

Half Reaction Method Balancing Redox Reactions In Basic Acidic 1) the balanced half reactions: 2) add for the final answer: note that the electrons were already balanced, so no need to multiply one or both half reactions by a factor. comment #1: notice that this is no h in the final answer, but please keep in mind that its presence is necessary for the reaction to proceed. Balance oxidation reduction reactions in neutral, acidic, or basic solutions using the half reaction method. in this section, we will focus on the half reaction method for balancing oxidation reduction reactions. This lesson introduces the step by step method for balancing redox reactions in basic solutions, as outlined in the ncert chemistry textbook for cbse class 11. Today, we will learn how to use the half cell method for balancing redox reactions in acidic and basic solutions. we will first balance a redox reaction in acidic solution, then we will balance the same redox reaction in basic solution.

Balancing Redox Reactions Occurring In Acidic Solution By The Half This lesson introduces the step by step method for balancing redox reactions in basic solutions, as outlined in the ncert chemistry textbook for cbse class 11. Today, we will learn how to use the half cell method for balancing redox reactions in acidic and basic solutions. we will first balance a redox reaction in acidic solution, then we will balance the same redox reaction in basic solution. In this article, we’ll learn about the half reaction method of balancing, a helpful procedure for balancing the equations of redox reactions occurring in aqueous solution. Do you have a redox equation you don't know how to balance? besides simply balancing the equation in question, these programs will also give you a detailed overview of the entire balancing process with your chosen method. As you delve deeper into redox reactions, it is essential to understand that balancing these reactions is critical for quantitative analysis in chemistry. an unbalanced equation might represent an incomplete process, leading to incorrect conclusions in experiments or industrial applications. Although the half reactions must be known to complete a redox reaction, it is often possible to figure them out without having to use a half reaction table. this is demonstrated in the acidic and basic solution examples.

Balancing Redox Reactions In Acidic Or Basic Solutions Course Hero In this article, we’ll learn about the half reaction method of balancing, a helpful procedure for balancing the equations of redox reactions occurring in aqueous solution. Do you have a redox equation you don't know how to balance? besides simply balancing the equation in question, these programs will also give you a detailed overview of the entire balancing process with your chosen method. As you delve deeper into redox reactions, it is essential to understand that balancing these reactions is critical for quantitative analysis in chemistry. an unbalanced equation might represent an incomplete process, leading to incorrect conclusions in experiments or industrial applications. Although the half reactions must be known to complete a redox reaction, it is often possible to figure them out without having to use a half reaction table. this is demonstrated in the acidic and basic solution examples.
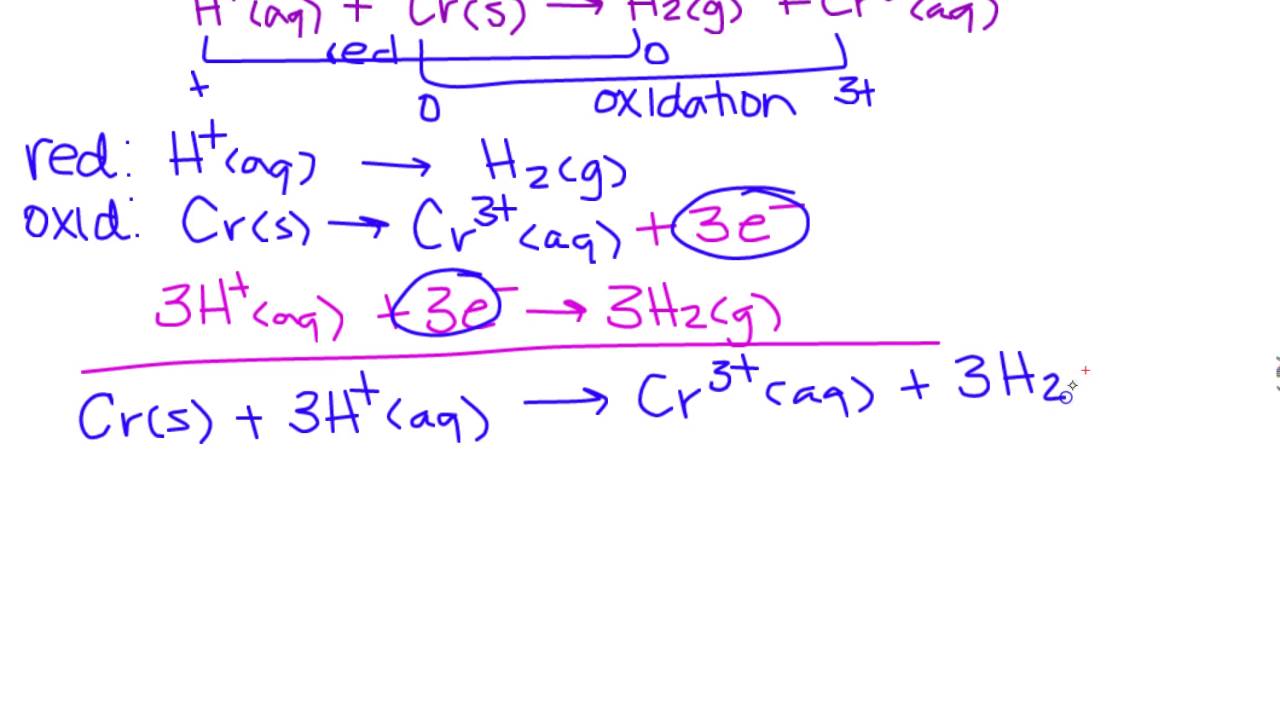
Balancing Redox Reactions In Acidic And Basic Conditions As you delve deeper into redox reactions, it is essential to understand that balancing these reactions is critical for quantitative analysis in chemistry. an unbalanced equation might represent an incomplete process, leading to incorrect conclusions in experiments or industrial applications. Although the half reactions must be known to complete a redox reaction, it is often possible to figure them out without having to use a half reaction table. this is demonstrated in the acidic and basic solution examples.

Half Reaction Method Balancing Redox Reactions Expii
Comments are closed.