How To Balance Redox Equations In Acidic Solution

How To Balance Redox Equations In Acidic Solution Video Summary And Q Oxidation reduction reactions, or redox reactions, are reactions in which one reactant is oxidized and one reactant is reduced simultaneously. this module demonstrates how to balance various redox …. Example #11: balance the equation for the reaction of stannous ion with pertechnetate in acidic solution. products are stannic ion, sn 4 and technetium (iv), tc 4 ions.

H2 Chemistry Strategy To Balance Redox Equations In Acidic Medium Today, we will learn how to use the half cell method for balancing redox reactions in acidic and basic solutions. we will first balance a redox reaction in acidic solution, then we will balance the same redox reaction in basic solution. We'll go step by step through how to balance an oxidation reduction (redox) reaction in acidic solution. most importantly, both charges and atoms must balance. Learn how to balance redox equations in acidic solution, and see examples that walk through sample problems step by step for you to improve your chemistry knowledge and skills. In this article, we’ll learn about the half reaction method of balancing, a helpful procedure for balancing the equations of redox reactions occurring in aqueous solution.
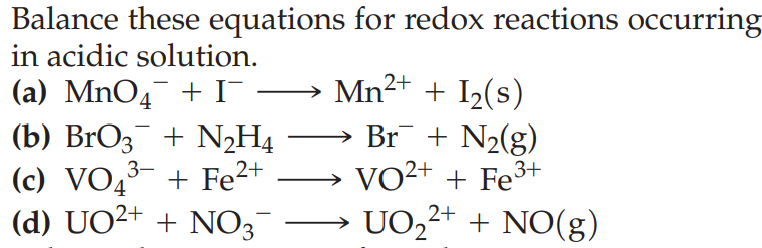
Solved Balance These Equations For Redox Reactions Occu Learn how to balance redox equations in acidic solution, and see examples that walk through sample problems step by step for you to improve your chemistry knowledge and skills. In this article, we’ll learn about the half reaction method of balancing, a helpful procedure for balancing the equations of redox reactions occurring in aqueous solution. In either situation we first balance the redox equations in acid solution. the product is water. oxidation half equation (acid solution) 1. add h₂o to balance o atoms. 2. balance h atoms with h . 3. balance charge with e⁻. there is 0 charge on lhs and 2 on rhs so add 2e to the rhs. 4. cancel h₂o. Once you know how to balance redox reaction equations in acidic solution, doing the same thing for basic solutions is not too difficult. here is the general procedure: begin by balancing the chemical equation with all the same steps as a reaction occurring in acidic solution. Redox reactions are significantly contextual, with distinctions arising based on whether they occur in acidic or basic environments. this article focuses specifically on the steps required to balance redox reactions in acidic solutions, which is a prevalent scenario in analytical chemistry. There will even be cases where balancing one half reaction using hydroxide can easily be done while the other half reaction gets balanced in acidic solution before converting.
Comments are closed.