How To Calculate Percent Composition Of Compounds General Chemistry

Percent Composition And Chemical Formulas Chemistry Studocu Below are the steps to find the percent composition of all the elements in a compound [1]. step 1: find the molar mass of all elements in the compound in terms of grams per mole (g mol 1). If you are studying a chemical compound, you may want to find the percent composition of a certain element within that chemical compound. the equation for percent composition is (mass of element molecular mass) x 100.
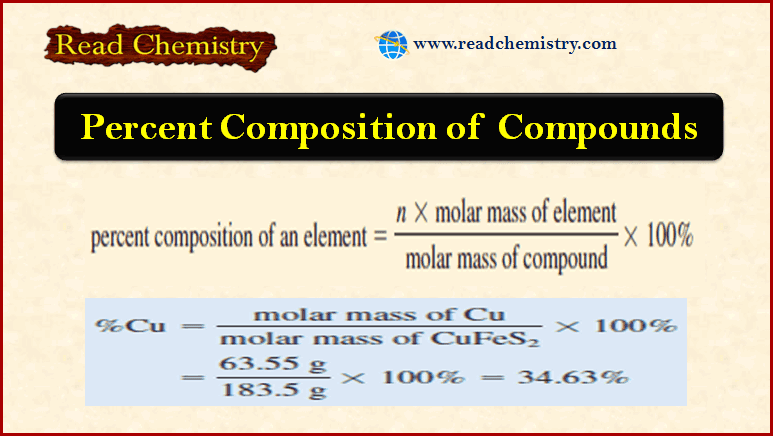
Percent Composition Of Compounds Read Chemistry The percentage composition of any given compound is defined as the ratio of the amount of each element to the total amount of all individual elements that are available in the given compound and then multiplied by 100. This article will explore percent composition, what is needed to determine percent composition, how to calculate it using the percent composition formula, and some frequently asked questions. what is percent composition?. To calculate the percentage of an element, multiply its molar mass and the subscript which shows how many moles of it are present in one mole of the compound, then divide the product by the molar mass of the compound. for example, let’s determine the percent composition of sulfuric acid (h 2 so 4). Learn how to calculate percentage composition (mass percent) with examples and simplify the process using our online percentage calculator for quick and accurate results.
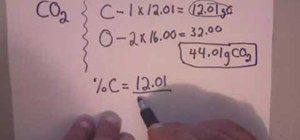
How To Calculate Percent Composition For Chemistry Math Wonderhowto To calculate the percentage of an element, multiply its molar mass and the subscript which shows how many moles of it are present in one mole of the compound, then divide the product by the molar mass of the compound. for example, let’s determine the percent composition of sulfuric acid (h 2 so 4). Learn how to calculate percentage composition (mass percent) with examples and simplify the process using our online percentage calculator for quick and accurate results. Percent composition quantifies the proportion of each element within a compound by mass. it is essential for analyzing chemical substances accurately. this article explores detailed formulas, common values, and real world applications of percent composition calculations. mastery of this concept is crucial for chemists and engineers. ¡hola!. To find the percent composition of each element in the compound, divide the total mass of each element by the molar mass of the compound and multiply by 100%. the formula for percent composition is:. Example: determine the % composition of each atom in glucose, c 6 h 12 o 6. steps to solve: 1. list all elements in the compound for which you would like to determine the percentage composition. c: h: o: 2. calculate the total mass of each element in the formula in small “chunks”. c: (6 atoms of c) x (12 g) = 72 g. Percent composition measures the relative quantities of each element in a compound. it represents the percentage, typically by mass, of each element compared to the total mass of the compound.
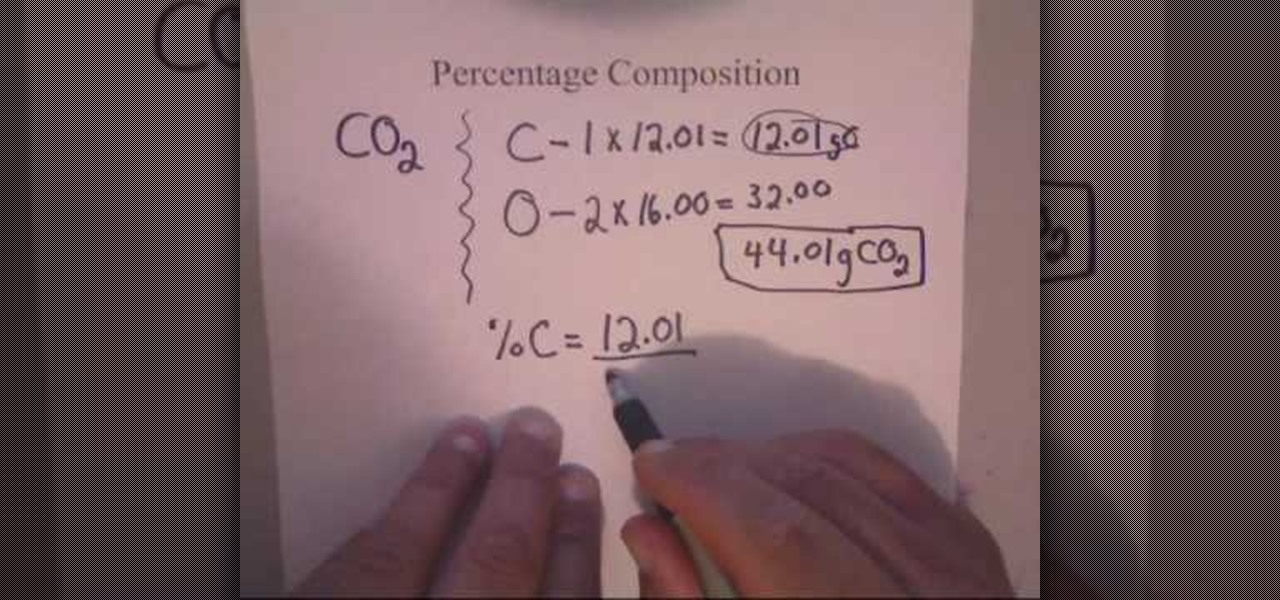
How To Calculate Percent Composition For Chemistry Math Wonderhowto Percent composition quantifies the proportion of each element within a compound by mass. it is essential for analyzing chemical substances accurately. this article explores detailed formulas, common values, and real world applications of percent composition calculations. mastery of this concept is crucial for chemists and engineers. ¡hola!. To find the percent composition of each element in the compound, divide the total mass of each element by the molar mass of the compound and multiply by 100%. the formula for percent composition is:. Example: determine the % composition of each atom in glucose, c 6 h 12 o 6. steps to solve: 1. list all elements in the compound for which you would like to determine the percentage composition. c: h: o: 2. calculate the total mass of each element in the formula in small “chunks”. c: (6 atoms of c) x (12 g) = 72 g. Percent composition measures the relative quantities of each element in a compound. it represents the percentage, typically by mass, of each element compared to the total mass of the compound.

Percent Composition Of Compounds Screencast Wisc Online Oer Example: determine the % composition of each atom in glucose, c 6 h 12 o 6. steps to solve: 1. list all elements in the compound for which you would like to determine the percentage composition. c: h: o: 2. calculate the total mass of each element in the formula in small “chunks”. c: (6 atoms of c) x (12 g) = 72 g. Percent composition measures the relative quantities of each element in a compound. it represents the percentage, typically by mass, of each element compared to the total mass of the compound.
Comments are closed.