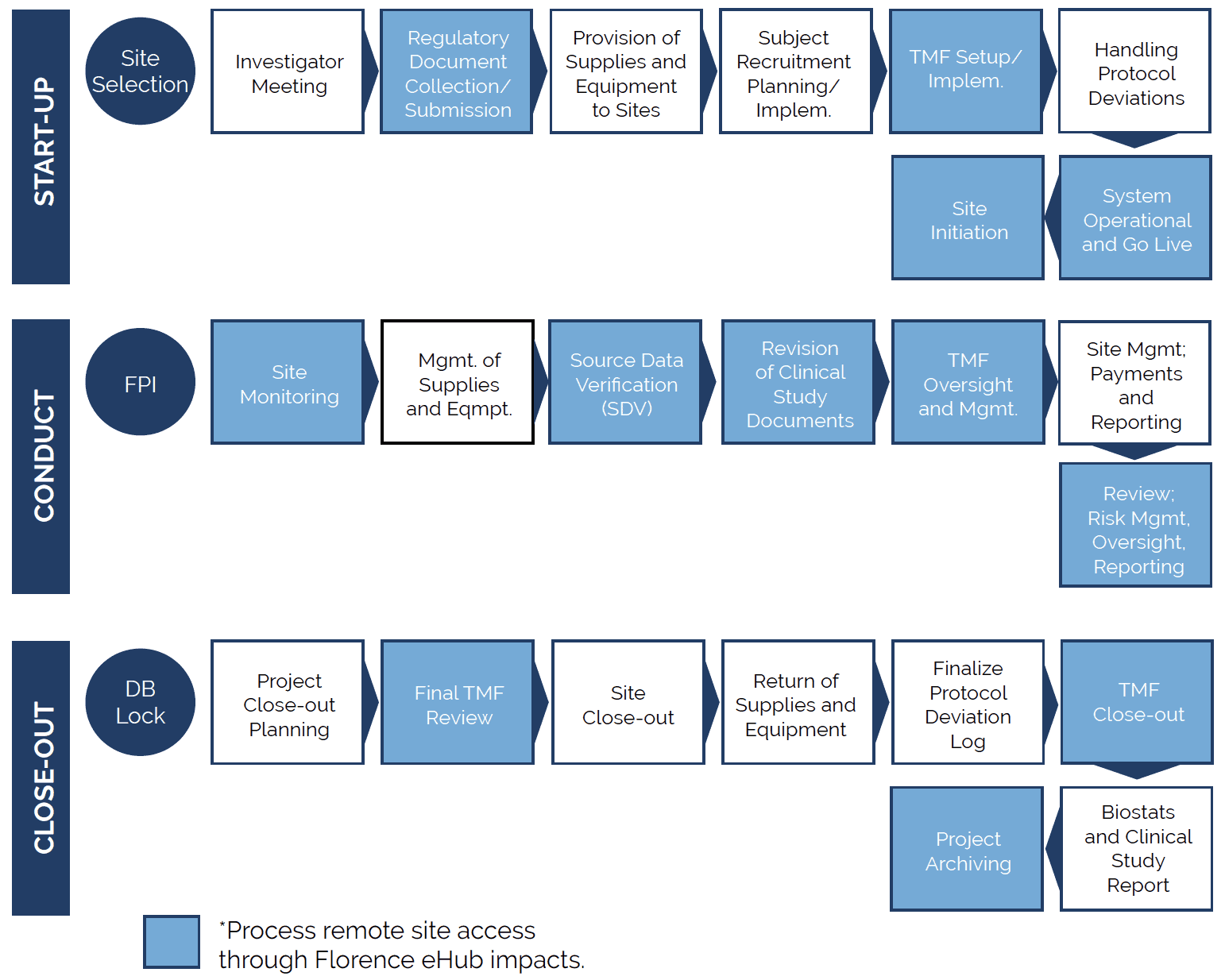
How To Create A Digitally Connected Clinical Trial Ecosystem Florence Creating seamless integration across sponsor, cro, and site digital platforms starts with examining the clinical trial processes that utilize each platform and identifying challenges within the current system. clinical studies typically follow three distinct phases: start up, conduct, and closeout. Gain insights from the clinical trial industry on technology adoption, integrating ai into site workflows, and how connectivity fosters collaboration among sites, sponsors and cros.
How To Create A Digitally Connected Clinical Trial Ecosystem Florence Clinical trials don’t have to come at a cost to the planet. by embracing digital first workflows, we can create a research ecosystem that’s faster, more equitable, and environmentally responsible. 🧠 ai in clinical trials: learn how artificial intelligence is revolutionizing recruitment, trial design, and data analysis. 🤝 bridging collaboration gaps: explore how sponsors, cros, and sites can overcome barriers and boost efficiency. Cognizant sip is the only open, collaborative clinical trial technology platform, leveraging one environment across multiple sponsors and operating via common workflows and documents. florence ebinders™ eisf helps sites save time by reducing paperwork and giving clinical trial sponsors instant remote access to a vast majority of their study. Digital technology has opened new possibilities for decentralised trials, which offer accelerated timelines, improved scalability and a more patient centric approach compared to traditional processes centred around hospitals.
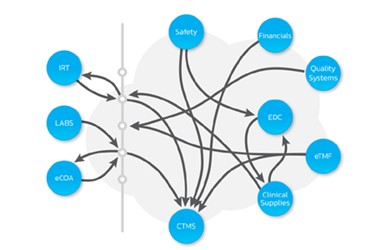
Data Governance In The Clinical Trial Ecosystem Cognizant sip is the only open, collaborative clinical trial technology platform, leveraging one environment across multiple sponsors and operating via common workflows and documents. florence ebinders™ eisf helps sites save time by reducing paperwork and giving clinical trial sponsors instant remote access to a vast majority of their study. Digital technology has opened new possibilities for decentralised trials, which offer accelerated timelines, improved scalability and a more patient centric approach compared to traditional processes centred around hospitals. By designing a platform around sites’ needs and enabling them to do their best work, florence frees clinical trials from bottlenecks, so they can accelerate and scale. examine the benefits of a site first approach to technology and consider how these tools are able to empower sites….

Digital Clinical Trial Ecosystem Services Hexaware By designing a platform around sites’ needs and enabling them to do their best work, florence frees clinical trials from bottlenecks, so they can accelerate and scale. examine the benefits of a site first approach to technology and consider how these tools are able to empower sites….
