In The Basic Cscl Crystal Structure Cs And Cl Ions Are Arranged Neet Physics Pyqs Electrostatic

Answered In The Basic Cscl Crystal Structure Cs And Cl Ions An Kunduz Solution: the given crystal structure is a body centered cubic structure. the electrostatic force. f = 4πε01 r2q1q2 due to one c s ion is balanced by diagonally opposite c s ion. hence, net electrostatic force on c l− ion due to eight c s ions is zero. In the basic cscl crystal structure cs and cl ions are arranged || neet physics pyqs electrostatic #jeephysics #neetphysics #neet33yearspreviousyearsque.

In The Basic Cscl Crystal Structure Cs And Cl Ions Are Arranged In A The given crystal structure is body centered cubic structure. the electrostatic force. f = 1 4 πε o q 1 q 2 r 2 due to one cs ion is balanced by diagonally opposite cs ion. hence net electrostatic on cl ion due to eight ions is zero. In the basic $cscl$ crystal structure, $cs^ $ and $cl^ $ ions are arranged in a bcc configuration as shown in the figure. the net electrostatic force exerted by the eight $cs^ { }$ ions on the $cl^ $ ions is. In the basic cscl crystal structure, cs and cl ions are arranged in a bcc configuration shown in the figure. the net electrostatic force exerted by the eight cs ions on the cl ion is [a] 14πε04e23a2 [b]14πε016e23a2 [c] 14πε032e23a2 [口] zero. your solution’s ready to go!. In the basic cscl crystal structure, cs and cl− ions are arranged in a bcc configuration as shown in the figure. the net electrostatic force exerted by the eight cs ions on the cl− ions is a.

In The Basic Cscl Crystal Structure Cs And Cl Ions Are Arranged In In the basic cscl crystal structure, cs and cl ions are arranged in a bcc configuration shown in the figure. the net electrostatic force exerted by the eight cs ions on the cl ion is [a] 14πε04e23a2 [b]14πε016e23a2 [c] 14πε032e23a2 [口] zero. your solution’s ready to go!. In the basic cscl crystal structure, cs and cl− ions are arranged in a bcc configuration as shown in the figure. the net electrostatic force exerted by the eight cs ions on the cl− ions is a. Cscl crystallize in a primitive cubic lattice which means the cubic unit cell has nodes only at its corners. the structure of cscl can be seen as two interpenetrating cubes, one of cs and one of cl . the ions are not touching one another. touching would cause repulsion between the anion and cation. The question and answers have been prepared according to the neet exam syllabus. information about in the basic cscl crystal structure cs and cl ion are arranged in a bcc configuration? covers all topics & solutions for neet 2025 exam. The electrostatic force due to one cs ion is balanced by diagonally opposite other cs . thus the net electrostatic force on cl ion due to eight cs ions is zero. The electrostatic force due to one cs ion is balanced by diagonally opposite other cs ion. thus, the net electrostatic force on cl− ion due to eight cs ions is zero.
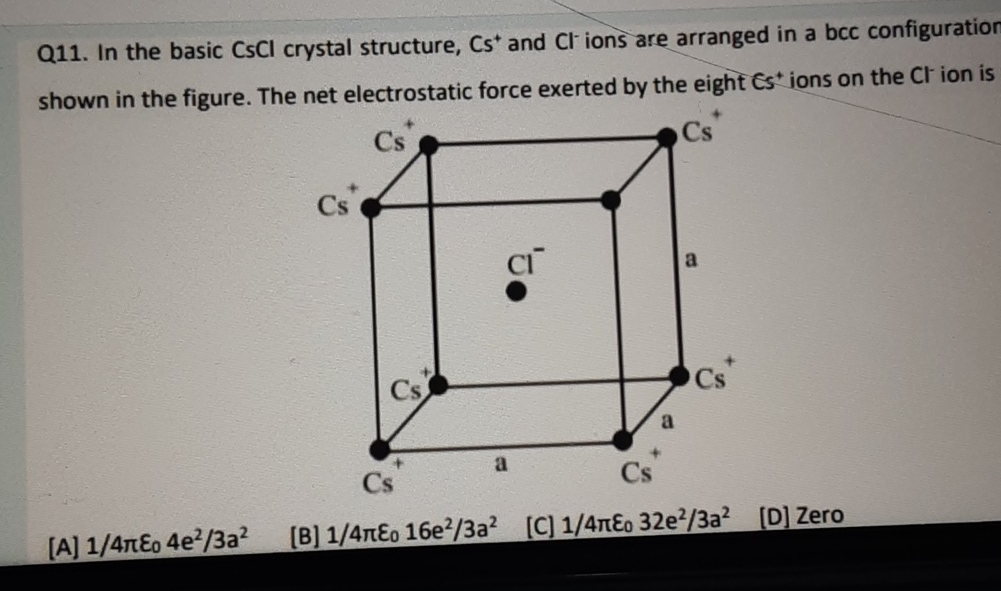
Solved Q11 ï In The Basic Cscl ï Crystal Structure Cs And Chegg Cscl crystallize in a primitive cubic lattice which means the cubic unit cell has nodes only at its corners. the structure of cscl can be seen as two interpenetrating cubes, one of cs and one of cl . the ions are not touching one another. touching would cause repulsion between the anion and cation. The question and answers have been prepared according to the neet exam syllabus. information about in the basic cscl crystal structure cs and cl ion are arranged in a bcc configuration? covers all topics & solutions for neet 2025 exam. The electrostatic force due to one cs ion is balanced by diagonally opposite other cs . thus the net electrostatic force on cl ion due to eight cs ions is zero. The electrostatic force due to one cs ion is balanced by diagonally opposite other cs ion. thus, the net electrostatic force on cl− ion due to eight cs ions is zero.

In The Basic Cscl Crystal Structure Cs And Cl Ions Are Arranged In A Bec The electrostatic force due to one cs ion is balanced by diagonally opposite other cs . thus the net electrostatic force on cl ion due to eight cs ions is zero. The electrostatic force due to one cs ion is balanced by diagonally opposite other cs ion. thus, the net electrostatic force on cl− ion due to eight cs ions is zero.
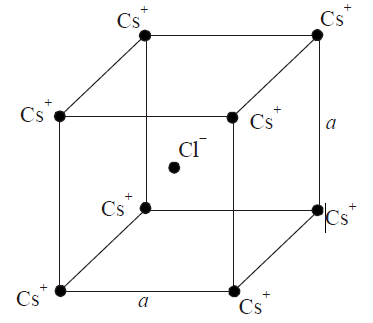
In The Basic Cscl Crystal Structure Cs And Cl Ions Are Ar
Comments are closed.