Pemigatinib Ema Panel Offers Conditional Nod For Pemigatinib

Pemigatinib A Review In Advanced Cholangiocarcinoma Adis Drug Pemazyre is approved based on tumor response and duration of response. there are ongoing studies to show whether pemazyre can improve survival or symptoms. your healthcare provider will test your cancer for a certain type of abnormal fgfr2 gene and make sure that pemazyre is right for you. Pemigatinib is used in adults to treat bile duct cancer that has spread to other parts of the body (metastatic) or cannot be removed with surgery. pemigatinib is usually given after your cancer has been treated with another medicine.
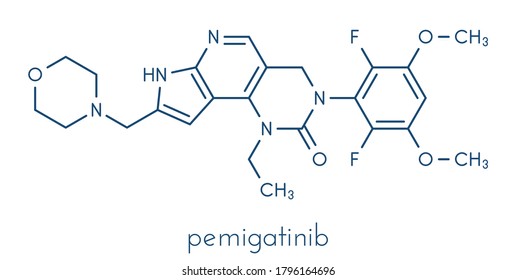
Pemigatinib Images Stock Photos Vectors Shutterstock Pemigatinib works by blocking the activity of abnormal fgfr2 proteins that signal cancer cells to divide. this keeps cancer cells from growing and may kill them. Pemigatinib, sold under the brand name pemazyre, is an anti cancer medication used for the treatment of bile duct cancer (cholangiocarcinoma). [5][6][8][9] pemigatinib works by blocking fgfr2 in tumor cells to prevent them from growing and spreading. Pemigatinib is used to treat cholangiocarcinoma (bile duct cancer) that has spread or cannot be removed by surgery in patients who have already tried cancer treatment before and have a certain type of abnormal fgfr2 gene in their cancer. your doctor will test for the presence of this gene. Pemigatinib is a small molecule kinase inhibitor that targets fgfr1, 2 and 3 with ic50 values of less than 2 nm. pemigatinib also inhibited fgfr4 in vitro at a concentration approximately 100.

Pemigatinib Uses Brand Names Mechanism Of Action Pemigatinib is used to treat cholangiocarcinoma (bile duct cancer) that has spread or cannot be removed by surgery in patients who have already tried cancer treatment before and have a certain type of abnormal fgfr2 gene in their cancer. your doctor will test for the presence of this gene. Pemigatinib is a small molecule kinase inhibitor that targets fgfr1, 2 and 3 with ic50 values of less than 2 nm. pemigatinib also inhibited fgfr4 in vitro at a concentration approximately 100. On april 17, 2020, the food and drug administration granted accelerated approval to pemigatinib (pemazyre, incyte corporation) for the treatment of adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma with a fibroblast growth factor receptor 2 (fgfr2) fusion or other rearrangement as detected by an fda a. Pemigatinib, a potent and selective small molecule inhibitor of fibroblast growth factor (fgf) receptors (fgfr1, fgfr2, and fgfr3), is an antineoplastic agent. Pemigatinib is a selective, potent, oral inhibitor of fgfr1, 2, and 3. this study evaluated the safety and antitumour activity of pemigatinib in patients with previously treated, locally advanced or metastatic cholangiocarcinoma with and without fgfr2 fusions or rearrangements. Pemigatinib is a type of targeted cancer drug. it is a treatment for bile duct cancer (cholangiocarcinoma). find out about how you have it, possible side effects and other important information.
Comments are closed.