Process And Reactions Occurring In A Iron Furnace

Mr Phillips Gcse Chemistry Iron Extraction And The Blast Furnace Carbon monoxide is generated in the hotter bottom regions and rises upward to reduce the iron oxides to pure iron through a series of reactions that take place in the upper regions. near the bottom of a furnace are nozzles through which preheated air is blown into the furnace. Use our notes to revise the extraction of iron from hematite for igcse chemistry. learn the reactions that occur in the blast furnace.
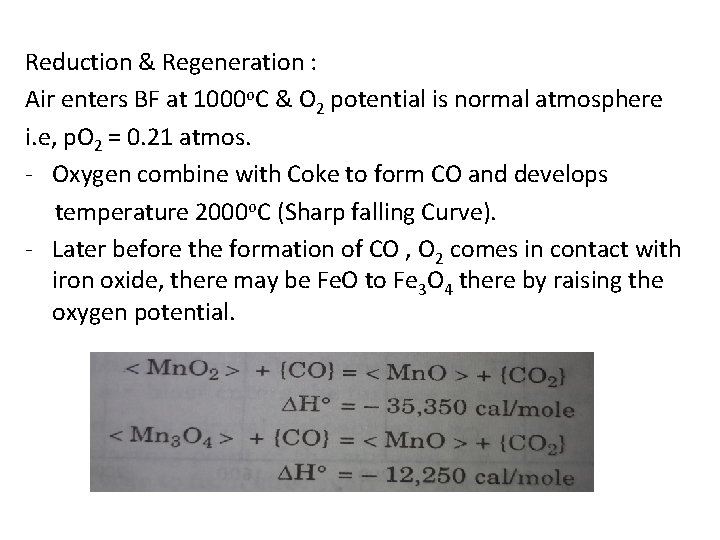
Iron Making Blast Furnace Reactions Basics Of Iron The term blast furnace comes from the blast of hot air that is blown into the lower part of the furnace at between 1400º to 2100ºf. molten iron is produced in a blast furnace by the following steps:. This in depth article explores the process of extraction of iron, focusing on the blast furnace method. it covers the essential raw materials, the chemical reactions involved, and the environmental impact of the process. Chemistry of the ironmaking by blast furnace process. the modern blast furnace (bf) operating with a low coke rate is an efficient processing unit primarily because of the intrinsic characteristics of a counter current gas solids reactor. Master iron extraction by blast furnace metallurgy. get clear explanations, key diagrams, and exam tips with vedantu.
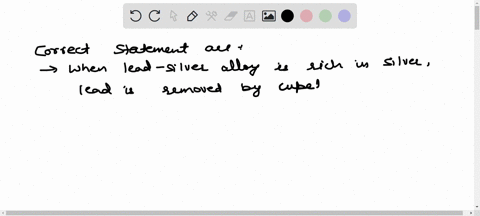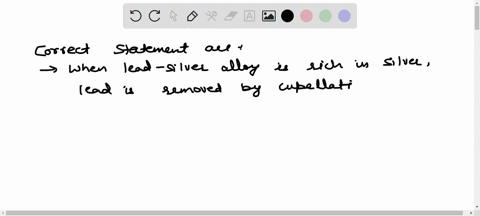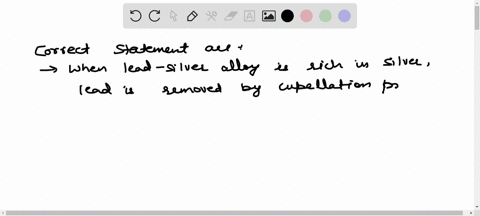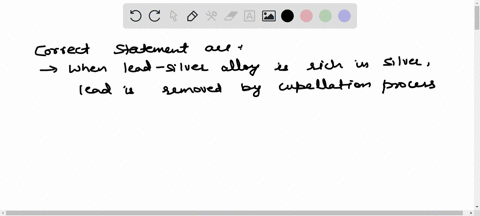
Solved The Chief Reaction S Occurring In Blast Furnace During Chemistry of the ironmaking by blast furnace process. the modern blast furnace (bf) operating with a low coke rate is an efficient processing unit primarily because of the intrinsic characteristics of a counter current gas solids reactor. Master iron extraction by blast furnace metallurgy. get clear explanations, key diagrams, and exam tips with vedantu. Improvements in the process over many centuries eventually led to the mass production of iron and to the industrial revolution. the reactions of the blast furnace involve 1) combustion of the fuel and its conversion into carbon monoxide, 2) reduction of the ore, and 3) formation of slag. In a blast furnace, fuel (coke), ores, and flux (limestone) are continuously supplied through the top of the furnace, while a hot blast of air (sometimes with oxygen enrichment) is blown into the lower section of the furnace through a series of pipes called tuyeres. Learn about redox, extraction of iron, and transition metals with bbc bitesize gcse chemistry (wjec). Fundamental studies based on bench scale reactors have highlighted the role of various mineral phases on the kinetics of gasification, hot metal carburization and slag reactions.
Comments are closed.