Redox Reaction Introduction And Discussion Of Theories Oxidation

Redox Reaction Pdf Redox Chemical Reactions To identify oxidation–reduction reactions in solution. the term oxidation was first used to describe reactions in which metals react with oxygen in air to produce metal oxides. when iron is exposed to air in the presence of water, for example, the iron turns to rust—an iron oxide. This important reaction class is defined by changes in oxidation states for one or more reactant elements, and it includes a subset of reactions involving the transfer of electrons between reactant species.
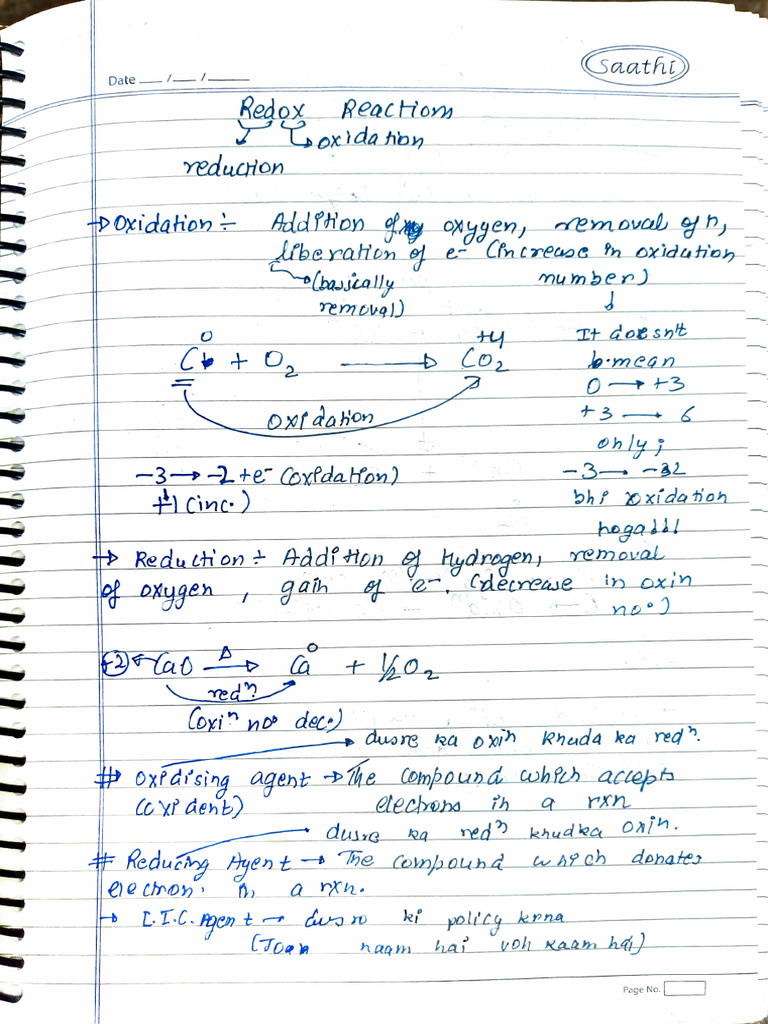
Redox Lecture 1 Pdf The document discusses redox (reduction oxidation) reactions, which involve the transfer of electrons between chemical species. it defines oxidation as the loss of electrons and reduction as the gain of electrons. it provides examples of redox reactions like the reaction between sodium and chlorine to form table salt. Oxidation and reduction reactions, commonly referred to as redox reactions, are truly ubiquitous in chemistry, underpinning processes in virtually every branch, from foundational inorganic and organic chemistry to intricate biochemical pathways and large scale industrial applications. In this chapter we will be introducing the chemistry of reduction oxidation (redox) reactions. this important reaction class is defined by changes in oxidation states for one or more reactant elements, and it includes a subset of reactions involving the transfer of electrons between reactant species. I. introduction to oxidation reduction (redox) reactions redox reactions are a major class of chemical reactions in which there is an exchange of electrons from one species to another.

Pptx Oxidation Reduction Redox Reactions 1 Redox Introduction In this chapter we will be introducing the chemistry of reduction oxidation (redox) reactions. this important reaction class is defined by changes in oxidation states for one or more reactant elements, and it includes a subset of reactions involving the transfer of electrons between reactant species. I. introduction to oxidation reduction (redox) reactions redox reactions are a major class of chemical reactions in which there is an exchange of electrons from one species to another. If any element undergoes a change of oxidation number in the course of a reaction, the reaction is a redox reaction. if an element’s oxidation number increases in a reaction, that element is oxidized. The introductory chapter on redox provides a comprehensive overview of oxidation and reduction processes, emphasizing their significance in various chemical reactions and biological systems. For the most part, when talking about redox reactions in organic chemistry we are dealing with a small set of very recognizable functional group transformations. it is therefore very worthwhile to become familiar with the idea of 'oxidation states' as applied to organic functional groups.
Comments are closed.