Revolutionizing Clinical Research Embracing Decentralized Trials In september 2024, the u.s. fda unveiled a final guidance aimed at advancing decentralized clinical trials (dcts), through its document conducting clinical trials with decentralized elements. this crucial regulatory framework outlines a pathway for integrating decentralized approaches into clinical research, emphasizing patient centricity, accessibility, and technological innovation. for. Decentralized clinical trials (dcts) have created a whole new chapter for clinical research trials. rather than requiring in person visits to specific doctor offices or research locations, dcts take a digital approach to collecting clinical data.

Decentralized Clinical Trials Technology In Clinical Trials Reshaping clinical trial conduct dcts represent a significant advancement in clinical research methodologies. by broadening patient demographics, enhancing trial diversity, and simplifying long term follow up, dcts offer a more inclusive and efficient approach to clinical research. Here we review the potential for digitally enabled and decentralized clinical trials to enhance clinical trial participation in an equitable manner. we describe the key barriers individuals. Decentralized clinical trials (dcts) are revolutionizing clinical research, offering potential improvements in trial efficiency and participant diversity. in a recent discussion, we explored these innovations with pamela tenaerts, chief scientific officer at medable, and ken getz, executive director and research professor at the tufts center. Background the importance of more diversity of study populations in clinical trials is currently widely acknowledged. decentralized clinical trial (dct) approaches are presented as a potential means to broaden diversity by eliminating several barriers to participation. however, the precise meaning of, and objectives related to diversity in dcts remain unclear. diversity runs the risk of.

Decentralized Clinical Trials Overcoming Challenges Embracing Decentralized clinical trials (dcts) are revolutionizing clinical research, offering potential improvements in trial efficiency and participant diversity. in a recent discussion, we explored these innovations with pamela tenaerts, chief scientific officer at medable, and ken getz, executive director and research professor at the tufts center. Background the importance of more diversity of study populations in clinical trials is currently widely acknowledged. decentralized clinical trial (dct) approaches are presented as a potential means to broaden diversity by eliminating several barriers to participation. however, the precise meaning of, and objectives related to diversity in dcts remain unclear. diversity runs the risk of. Decentralized clinical trials are revolutionizing the landscape of clinical research, offering unprecedented opportunities to enhance efficiency, inclusivity, and patient centricity. Summary the clinical trials market is undergoing a transformative shift driven by ai integration, decentralized models, and a surge in global research investments. from virtual trials to patient centric designs, the industry is poised for exponential growth. In this review, we explore the transformative potential of decentralized clinical trials (dcts) in addressing the limitations of traditional randomized controlled trials (rcts). we highlight the merits of dcts fostering greater inclusivity, efficiency, and adaptability. Decentralized clinical trials (dcts) are revolutionizing the clinical research landscape by leveraging technology to improve patient access and data management. as trials become more patient centric, decentralized models offer new opportunities for enhancing efficiency and improving data collection.

Decentralized Clinical Trials Growing Trend Tips And Best Practices Decentralized clinical trials are revolutionizing the landscape of clinical research, offering unprecedented opportunities to enhance efficiency, inclusivity, and patient centricity. Summary the clinical trials market is undergoing a transformative shift driven by ai integration, decentralized models, and a surge in global research investments. from virtual trials to patient centric designs, the industry is poised for exponential growth. In this review, we explore the transformative potential of decentralized clinical trials (dcts) in addressing the limitations of traditional randomized controlled trials (rcts). we highlight the merits of dcts fostering greater inclusivity, efficiency, and adaptability. Decentralized clinical trials (dcts) are revolutionizing the clinical research landscape by leveraging technology to improve patient access and data management. as trials become more patient centric, decentralized models offer new opportunities for enhancing efficiency and improving data collection.
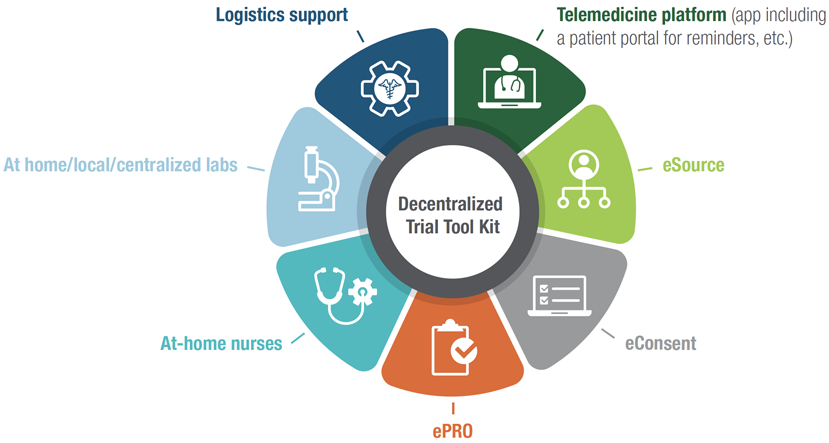
The Technology Surrounding Decentralized Clinical Trials In this review, we explore the transformative potential of decentralized clinical trials (dcts) in addressing the limitations of traditional randomized controlled trials (rcts). we highlight the merits of dcts fostering greater inclusivity, efficiency, and adaptability. Decentralized clinical trials (dcts) are revolutionizing the clinical research landscape by leveraging technology to improve patient access and data management. as trials become more patient centric, decentralized models offer new opportunities for enhancing efficiency and improving data collection.
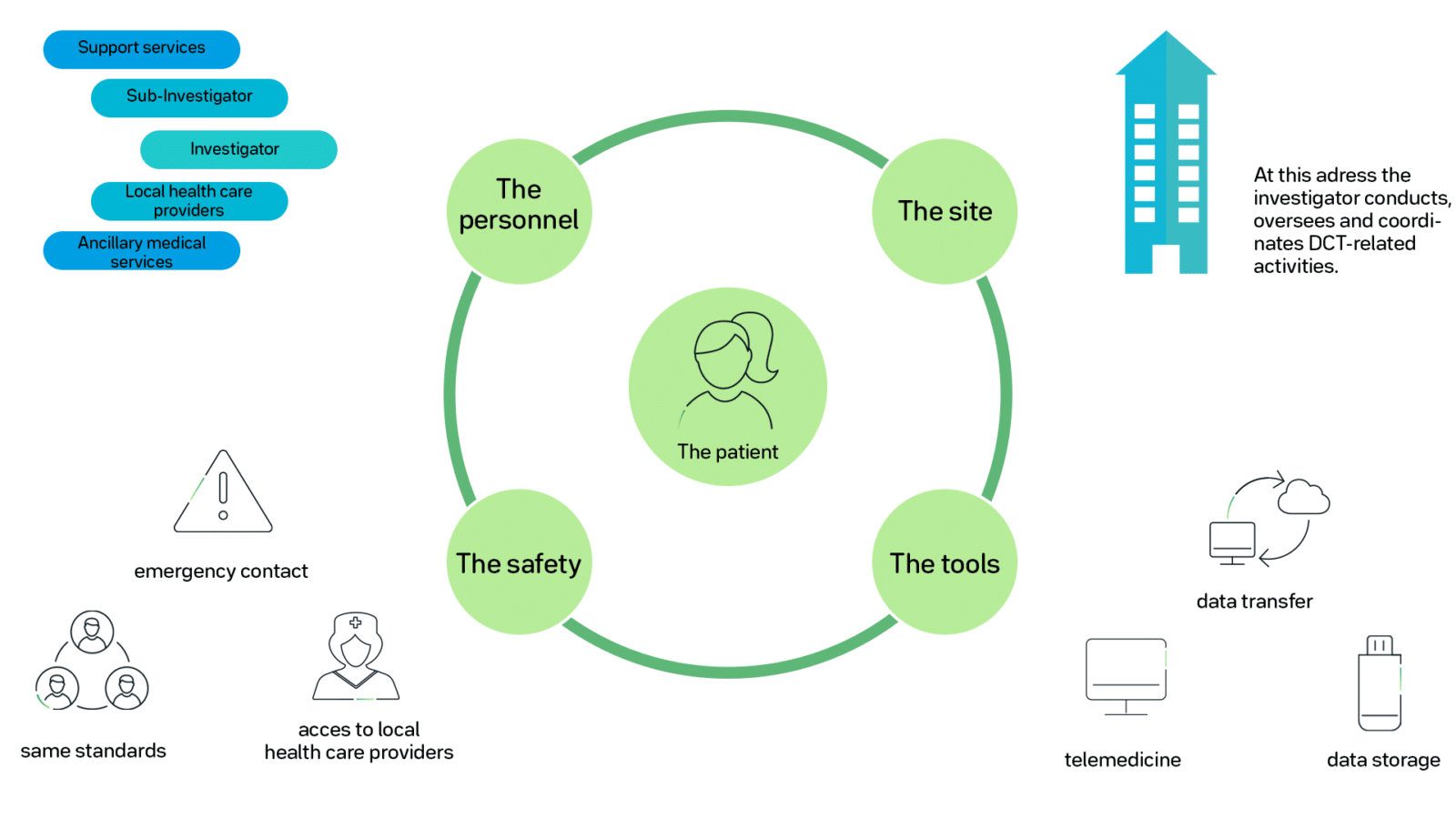
Decentralized Clinical Trials In The Times Of Modern Technology
