Sample Problem 8 3 Identifying Bond Type Ionic Vs Covalent Character

Covalent Character In An Ionic Bond Occurs Pdf Playlist: • chemical bonds playlist: • chemistry chapter 8, sample problem 8.3: which type of bond (nonpolar covalent, moderately polar covalent, very polar covalent, or ionic). Ionic bonds form when one atom transfers electrons to another, essentially donating or receiving electrons to achieve stability. in contrast, covalent bonds happen when atoms share electrons, working together to complete their outer electron shells.
Ionic And Covalent Bond Match Up Problem \ (\pageindex {2}\) using the periodic table, predict whether the following chlorides are ionic or covalent: sicl 4, pcl 3, cacl 2, cscl, cucl 2, and crcl 3. These worksheets are meant to test what a student knows about ionic and covalent bonding. they should be able to tell the difference between the two. suitable for: grade 7, grade 8, grade 9, grade 10, grade 11, grade 12. Learn the difference between ionic and covalent bonds. see examples of the two types of chemical bonding and how to predict which type of bond will form. 6) magnesium phosphide (mg and p) formula: bonding in the following pairs of elements. once you have determined the structure for the molecule, write its structural formula in the space provided; use a dash to represent a shared pair of e.
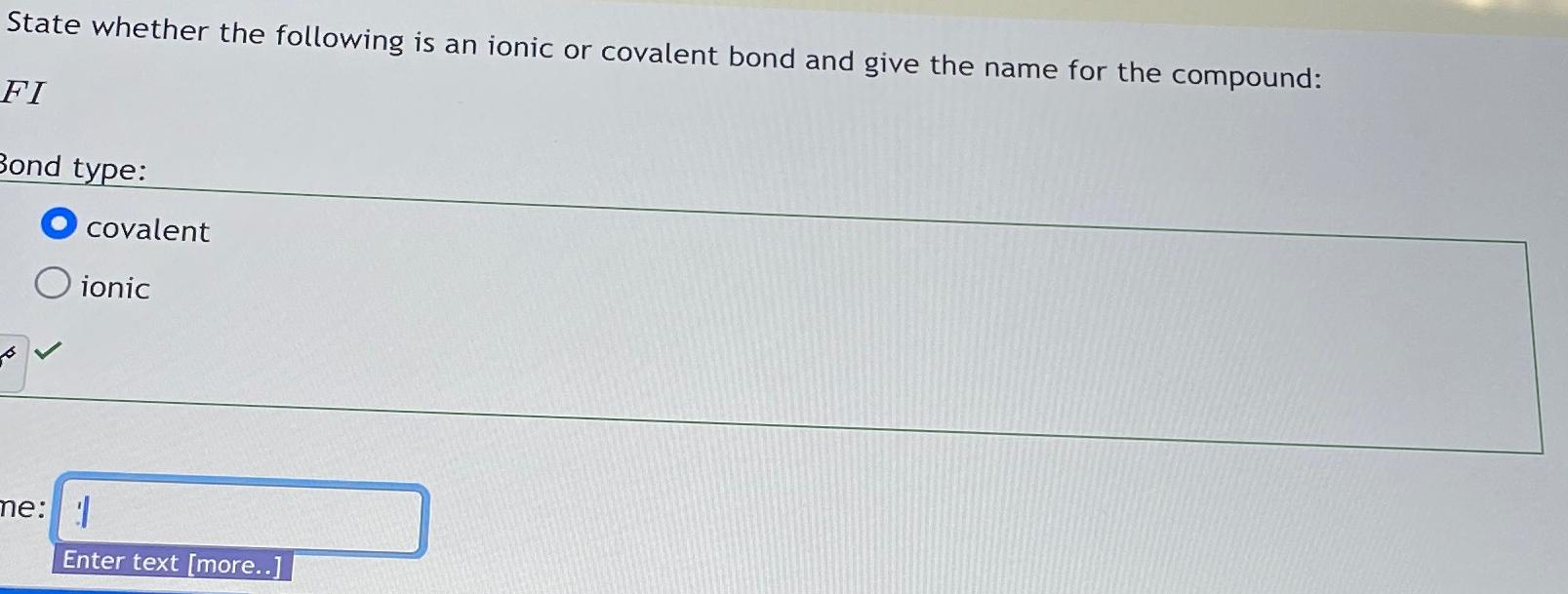
Solved State Whether The Following Is An Ionic Or Covalent Chegg Learn the difference between ionic and covalent bonds. see examples of the two types of chemical bonding and how to predict which type of bond will form. 6) magnesium phosphide (mg and p) formula: bonding in the following pairs of elements. once you have determined the structure for the molecule, write its structural formula in the space provided; use a dash to represent a shared pair of e. Ionic compounds form in strict ratios of anions to cations to gain overall neutrality. for example, ca2 and cl will form an ionic lattice in the ratio 1 : 2 (1 × 2 2 × ( 1) = 0). Use your understanding of chemical bonds to determine whether each of the chemical formulas that are listed are ionic or covalent. There is a handout included for students to fill in and summarize the difference between ionic and covalent bonds. this is an important handout for students to use as a quick reference in the future. If you sent a sample of this compound to a commercial laboratory for elemental analysis, what results would you expect for the mass percentages of carbon, hydrogen, and nitrogen?.

Solved 24 Determine The Type Of Bond Ionic Bond Polar Covalent Bond Ionic compounds form in strict ratios of anions to cations to gain overall neutrality. for example, ca2 and cl will form an ionic lattice in the ratio 1 : 2 (1 × 2 2 × ( 1) = 0). Use your understanding of chemical bonds to determine whether each of the chemical formulas that are listed are ionic or covalent. There is a handout included for students to fill in and summarize the difference between ionic and covalent bonds. this is an important handout for students to use as a quick reference in the future. If you sent a sample of this compound to a commercial laboratory for elemental analysis, what results would you expect for the mass percentages of carbon, hydrogen, and nitrogen?.

Solved 18 Determine The Type Of Bond Ionic Bond Polar Chegg There is a handout included for students to fill in and summarize the difference between ionic and covalent bonds. this is an important handout for students to use as a quick reference in the future. If you sent a sample of this compound to a commercial laboratory for elemental analysis, what results would you expect for the mass percentages of carbon, hydrogen, and nitrogen?.
Comments are closed.