
Unsaturated Saturated And Supersaturated Solutions Define saturated. define unsaturated. apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. define supersaturated. explain how supersaturated solutions are created. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: saturated, unsaturated, and supersaturated. saturated solution: a saturated solution is a solution in which the maximum amount of solute has been dissolved in a given amount of solvent at a particular temperature.
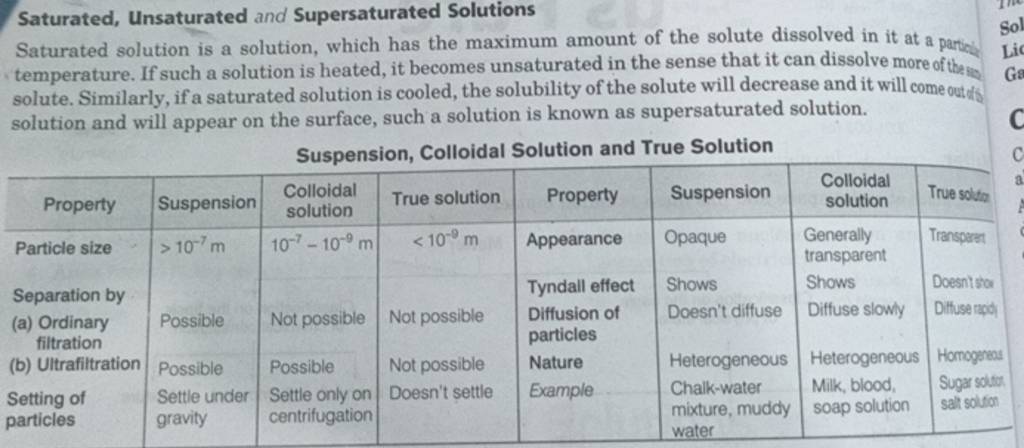
Saturated Unsaturated And Supersaturated Solutions Saturated Solution Is Saturated solution: it's a chemical solution that has reached its maximum solute concentration, where no additional solute can dissolve at a specific temperature. supersaturated solution: this is a state beyond saturation, where a solution temporarily holds more dissolved solute than it would under normal circumstances. The key difference between a saturated solution and an unsaturated solution is that in a saturated solution, the dissolved solute is the maximum amount of solute, a solvent can dissolve at a given temperature and pressure, while in an unsaturated solution, we can dissolve more solute in solvent. Saturated solution: there is so much solute present that if more were added, it would not dissolve. unsaturated solution: contains less solute than a saturated solution which completely dissolves leaving no remaining substances. supersaturated solution: a solution that contains more solute than the solvent is capable of dissolving. A saturated solution has the maximum amount of solute dissolved at a specific temperature, while a supersaturated solution contains more solute than what would normally be possible at that temperature.

Saturation Video Chemistry Ck 12 Foundation Saturated solution: there is so much solute present that if more were added, it would not dissolve. unsaturated solution: contains less solute than a saturated solution which completely dissolves leaving no remaining substances. supersaturated solution: a solution that contains more solute than the solvent is capable of dissolving. A saturated solution has the maximum amount of solute dissolved at a specific temperature, while a supersaturated solution contains more solute than what would normally be possible at that temperature. Based on the solubility of a substance, a solution can be classified as saturated, unsaturated, or supersaturated. a saturated solution is a solution in which no more solute can dissolve in the solvent at a given temperature and pressure. this means that the concentration of the solute in the solution is at its maximum limit. Unsaturated solution is a solution where more solute can be added to the solution at a constant temperature. in other words, there is still room for more solute to dissolve in the solvent. unsaturated solutions can be created by adding less solute than the solubility limit or by lowering the temperature, which reduces the solubility of the solute. Note : a supersaturated solution contains more than the maximum amount of solute that can be dissolved at that temperature. it is unstable and the solute will usually begin to crystallize, especially if disturbed. example : a solution of 40g salt in 100 g water is super saturated solution. Then, we will learn how to tell whether a solution is saturated, unsaturated, and supersaturated based on solubility curves. next, we will make a table showing the differences between these three types of solutions. lastly, we will explore some examples of saturated, unsaturated, and supersaturated solutions.

Saturated Unsaturated And Supersaturated Solution Chemistry Go It Based on the solubility of a substance, a solution can be classified as saturated, unsaturated, or supersaturated. a saturated solution is a solution in which no more solute can dissolve in the solvent at a given temperature and pressure. this means that the concentration of the solute in the solution is at its maximum limit. Unsaturated solution is a solution where more solute can be added to the solution at a constant temperature. in other words, there is still room for more solute to dissolve in the solvent. unsaturated solutions can be created by adding less solute than the solubility limit or by lowering the temperature, which reduces the solubility of the solute. Note : a supersaturated solution contains more than the maximum amount of solute that can be dissolved at that temperature. it is unstable and the solute will usually begin to crystallize, especially if disturbed. example : a solution of 40g salt in 100 g water is super saturated solution. Then, we will learn how to tell whether a solution is saturated, unsaturated, and supersaturated based on solubility curves. next, we will make a table showing the differences between these three types of solutions. lastly, we will explore some examples of saturated, unsaturated, and supersaturated solutions.

Ppt Saturated Unsaturated Supersaturated Powerpoint Presentation Note : a supersaturated solution contains more than the maximum amount of solute that can be dissolved at that temperature. it is unstable and the solute will usually begin to crystallize, especially if disturbed. example : a solution of 40g salt in 100 g water is super saturated solution. Then, we will learn how to tell whether a solution is saturated, unsaturated, and supersaturated based on solubility curves. next, we will make a table showing the differences between these three types of solutions. lastly, we will explore some examples of saturated, unsaturated, and supersaturated solutions.
