Solution Different Types Of Solution With Examples Studypool
Solution Definition Types Properties Examples And Faqs 43 Off For example, suppositories are intended for introduction into the rectum or vagina, and they are produced in the solid dosage form that melts at body temperature. In all solutions, whether gaseous, liquid, or solid, the substance present in the greatest amount is the solvent, and the substance or substances present in lesser amounts are the solute (s).

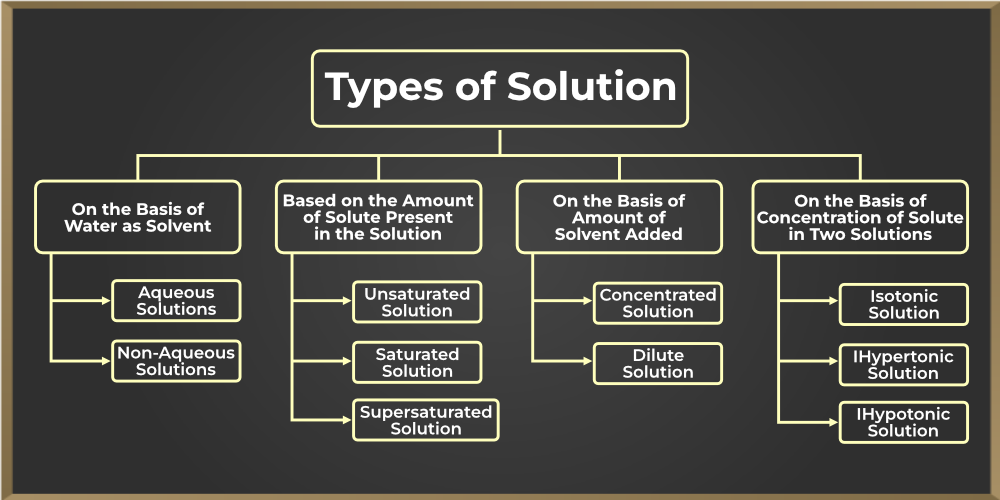
Example 2 Solution Pdf Types of solutions a solution is a homogeneous mixture of two substances: a solute and a solvent. solute: substance being dissolved; present in lesser amount. solvent: substance doing the dissolving; present in larger amount. solutes and solvents may be of any form of matter: solid, liquid or gas. In chemistry, the term “solution” refers to a homogeneous mixture of two or more substances in relative amounts. although the term “solution” refers to the liquid state of matter, solutions of gases and solids are also possible. Examples of homogeneous solutions are a cup of coffee, perfume, salt, or sugar in water, etc., and the examples of heterogeneous solutions are a solution of oil and water, a solution of sand and water, a solution of chalk powder, and water, etc. For example, a mixture of nitrogen and oxygen gases, an aqueous solution of sodium chloride or carbon dioxide, and a mixture of sodium and mercury in sodium amalgam. solutions have two parts solute and solvent. the ability of a solute to dissolve in a solvent is called solubility.

Solution Different Types Of Solution With Examples Studypool Examples of homogeneous solutions are a cup of coffee, perfume, salt, or sugar in water, etc., and the examples of heterogeneous solutions are a solution of oil and water, a solution of sand and water, a solution of chalk powder, and water, etc. For example, a mixture of nitrogen and oxygen gases, an aqueous solution of sodium chloride or carbon dioxide, and a mixture of sodium and mercury in sodium amalgam. solutions have two parts solute and solvent. the ability of a solute to dissolve in a solvent is called solubility. During the formation of a solution, any state of matter (solid, liquid, or gas) can act as both a solvent and a solute. as a result, depending on the physical states of the solute and solvent, there are nine different types of solutions:. 6.3 types of solution as we know that there are three states of matter, i.e. solid, liquid and gas. a solute as well as solvent can exist in any one of the three states of matter. by the combination of these three states different types of solutions are formed, which are given in table 6.2. In this article we will discuss aqueous solution, types of solution, homogeneous solution, homogeneous solution examples and get detailed information including the different types, heterogeneous, examples, faqs and more here. These differences in conductivity between different types of strong electrolytes can sometimes be very useful in deciding what ions are actually present in a given electrolyte solution as the following example makes clear.

Solution Strategies For Different Types Of Problems Download During the formation of a solution, any state of matter (solid, liquid, or gas) can act as both a solvent and a solute. as a result, depending on the physical states of the solute and solvent, there are nine different types of solutions:. 6.3 types of solution as we know that there are three states of matter, i.e. solid, liquid and gas. a solute as well as solvent can exist in any one of the three states of matter. by the combination of these three states different types of solutions are formed, which are given in table 6.2. In this article we will discuss aqueous solution, types of solution, homogeneous solution, homogeneous solution examples and get detailed information including the different types, heterogeneous, examples, faqs and more here. These differences in conductivity between different types of strong electrolytes can sometimes be very useful in deciding what ions are actually present in a given electrolyte solution as the following example makes clear.

Solution Types Of Solution Studypool In this article we will discuss aqueous solution, types of solution, homogeneous solution, homogeneous solution examples and get detailed information including the different types, heterogeneous, examples, faqs and more here. These differences in conductivity between different types of strong electrolytes can sometimes be very useful in deciding what ions are actually present in a given electrolyte solution as the following example makes clear.
Comments are closed.