Solution Lecture 12 Entropy And Gibbs Free Energy 2023 35slides 3h

Entropy Enthalpy Gibbs Free Energy Pdf Entropy Gibbs Free Energy Δs = qrev t entropy and gibbs free energy entropy entropy always increases by increasing the temperature. – entropy is a state function whose variation depends only on the initial state and the final state, but not on the path used to move from one to the other. Lecture 12 covers the fundamentals of metabolism, emphasizing thermodynamics, enzyme function, and metabolic regulation. key concepts include the dynamics of living systems, the roles of catabolism and anabolism, and the coupling of reactions to drive unfavorable processes.
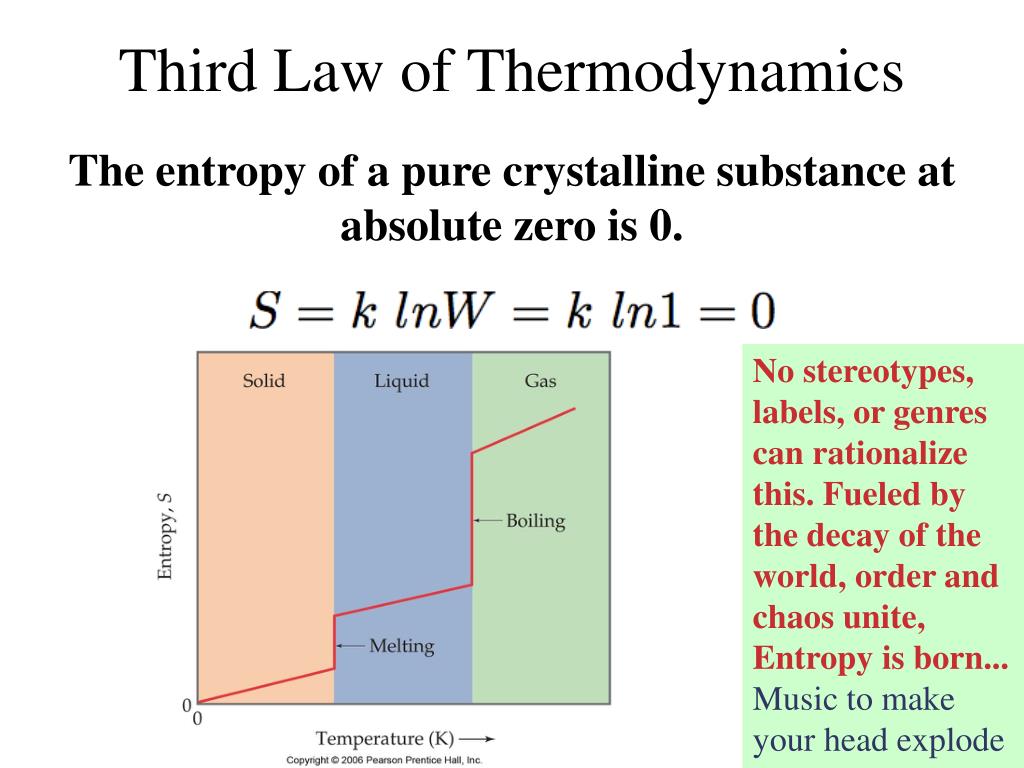
Ppt Entropy Gibbs Free Energy Powerpoint Presentation Free Gibbs free energy (g) takes into account both enthalpy and entropy to determine if a reaction is spontaneous. a reaction is spontaneous if the change in gibbs free energy (Δg) is negative. Chapter 12: entropy and gibbs free energy ch12.1 entropy ch12.2 entropy and microstates ch12.3 predicting the sign of Δs ch12.4 second law of thermodynamics. Ct 5. hess’s law: flip equations, multiply coefficients, add equations, etc. we can apply method’s 3 5 to entropy, Δs, and gibb’s free energy, Δg, calculations. for example: the “big mamma equation” can be applied to Δs and Δg. ∆s = ∑ s°(products) − ∑ s°(reactants) ∆g° = ∑ ∆ g°f (products) − ∑ ∆ g°f. Live explanations & solutions for entropy and gibbs free energy questions from friendly tutors over 1:1 instant tutoring sessions. ask for solutions, concepts, examples or practice problems.

Solved Enthalpy Entropy And Gibbs Free Energy The Relation Chegg Ct 5. hess’s law: flip equations, multiply coefficients, add equations, etc. we can apply method’s 3 5 to entropy, Δs, and gibb’s free energy, Δg, calculations. for example: the “big mamma equation” can be applied to Δs and Δg. ∆s = ∑ s°(products) − ∑ s°(reactants) ∆g° = ∑ ∆ g°f (products) − ∑ ∆ g°f. Live explanations & solutions for entropy and gibbs free energy questions from friendly tutors over 1:1 instant tutoring sessions. ask for solutions, concepts, examples or practice problems. Some of the energy was utilized to compensate the loss in entropy. was this document helpful? conditions?. A summary practice problem set on chemical thermodynamics to cover examples of entropy changes, free energy, correlating Δg, Δs and Δh. Gibbs free energy combines entropy and enthalpy, with spontaneous reactions having a negative change in gibbs free energy. the document provides examples to illustrate these concepts. This chemistry chapter provides video lessons on gibbs free energy, entropy, enthalpy, spontaneity formulas, and the 2nd law of thermodynamics.
Comments are closed.