Solved 1 Calculate The Molarity Of Each Of The Following Chegg
Solved Calculate The Molarity Of Each Of The Following Chegg To find the molarity of a 60.0% solution of c h 3 c o o h with a specific gravity of 1.05, calculate the molar mass of c h 3 c o o h by adding the atomic masses of its constituent elements. Since the molar amount of solute and the volume of solution are both given, the molarity can be calculated using the definition of molarity. per this definition, the solution volume must be converted from ml to l: a teaspoon of table sugar contains about 0.01 mol sucrose.
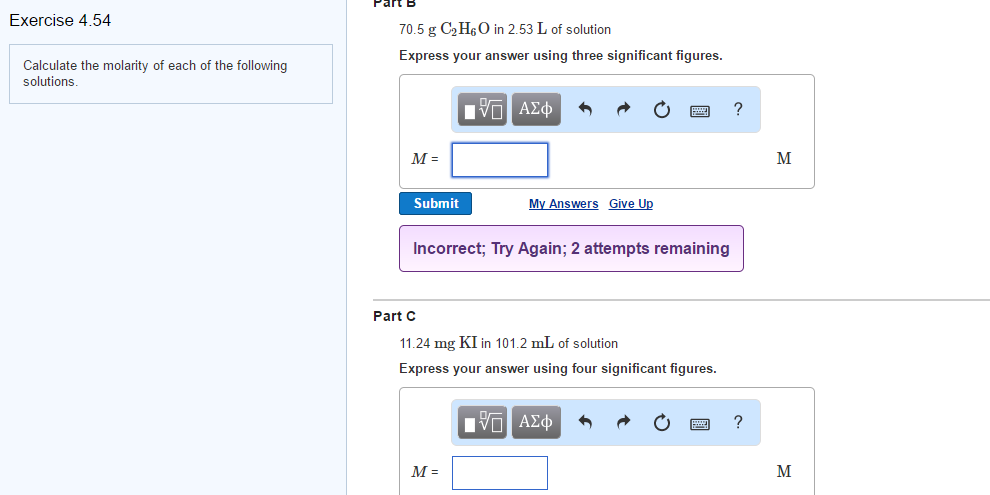
Solved Calculate The Molarity Of The Following Solutions Chegg Calculate the molarity of each of the following solutions: (a) 0.195 g of cholesterol, c h o, in 0.100 l of serum, the average concentration of cholesterol in human serum. Find step by step chemistry solutions and your answer to the following textbook question: calculate the molarity of each of the following solutions: (a) 0.195 g of cholesterol, $\mathrm {c} {27} \mathrm {h} {46} \mathrm {o}$, in 0.100 l of serum, the average concentration of cholesterol in human serum (b) 4.25 g of $\mathrm {nh} {3}$ in 0. Step 3: finally, we calculate the molarity of each solution using the formula: molarity = number of moles volume of the solution in liters assuming the volume of the solution is 1 l for each compound, the molarity for c2h5br is 0.266 mol 1 l = 0.266 m. the molarity for na2s2o3 is 0.139 mol 1 l = 0.139 m. Finally, calculate the molarity of each solution using its formula: molarity = moles of solute volume of solution in liters. thus, the molarity of each solution is: (a) 0.205 m o l 1.50 l = 0.137 m, (b) 0.094 m o l 2.20 l = 0.043 m, and (c) 0.061 m o l 0.0852 l = 0.716 m.

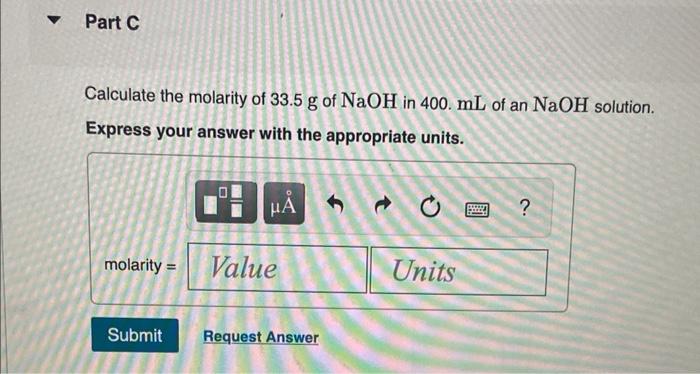
Solved Calculate The Molarity Of The Following Solutions Chegg Step 3: finally, we calculate the molarity of each solution using the formula: molarity = number of moles volume of the solution in liters assuming the volume of the solution is 1 l for each compound, the molarity for c2h5br is 0.266 mol 1 l = 0.266 m. the molarity for na2s2o3 is 0.139 mol 1 l = 0.139 m. Finally, calculate the molarity of each solution using its formula: molarity = moles of solute volume of solution in liters. thus, the molarity of each solution is: (a) 0.205 m o l 1.50 l = 0.137 m, (b) 0.094 m o l 2.20 l = 0.043 m, and (c) 0.061 m o l 0.0852 l = 0.716 m. Calculate mass required for molar solution the molarity calculator calculates the mass of compound required to achieve a specific molar concentration and volume. to dilute a solution of known molarity, please use the solution dilution calculator. to dilute a solution of concentrated acid or base of known w w% strength, please use the acid & base molarity calculator. 1. calculate the molarity of each of the following solutions. start from your mass of solute in numerator and mass of solution in the denominator. use molar mass to change from grams to moles of solute in numerator. use the density you calculated to change from mass of solution to volume of solution in denominator. show one sample calculation. Since the molar amount of solute and the volume of solution are both given, the molarity can be calculated using the definition of molarity. per this definition, the solution volume must be converted from ml to l: a teaspoon of table sugar contains about 0.01 mol sucrose. The following paragraphs will present and apply the equation that is used to determine the molarity of a solution, which, in contrast to the previously discussed concentrations, is not calculated as a percentage.

Solved 1 Calculate The Molarity Of Each Of The Following Chegg Calculate mass required for molar solution the molarity calculator calculates the mass of compound required to achieve a specific molar concentration and volume. to dilute a solution of known molarity, please use the solution dilution calculator. to dilute a solution of concentrated acid or base of known w w% strength, please use the acid & base molarity calculator. 1. calculate the molarity of each of the following solutions. start from your mass of solute in numerator and mass of solution in the denominator. use molar mass to change from grams to moles of solute in numerator. use the density you calculated to change from mass of solution to volume of solution in denominator. show one sample calculation. Since the molar amount of solute and the volume of solution are both given, the molarity can be calculated using the definition of molarity. per this definition, the solution volume must be converted from ml to l: a teaspoon of table sugar contains about 0.01 mol sucrose. The following paragraphs will present and apply the equation that is used to determine the molarity of a solution, which, in contrast to the previously discussed concentrations, is not calculated as a percentage.

Solved 1 Calculate The Molarity Of Each Of The Following Chegg Since the molar amount of solute and the volume of solution are both given, the molarity can be calculated using the definition of molarity. per this definition, the solution volume must be converted from ml to l: a teaspoon of table sugar contains about 0.01 mol sucrose. The following paragraphs will present and apply the equation that is used to determine the molarity of a solution, which, in contrast to the previously discussed concentrations, is not calculated as a percentage.

Solved 1 Calculate The Molarity Of Each Of The Following Chegg
Comments are closed.