Solved 1 Consider The Following Two Molecules Which Would Chegg

Solved Consider The Following Molecules Select All The Chegg Consider the following two molecules: a) draw the two possible chair conformations for molecule a. (2 points) b) which chair conformation in part a is most stable and why? (2 points) c) draw the two possible chair conformations for molecule b. (2 points) d) which chair conformation in part c is more stable and why? (2 points) e) which molecule. H e2 has two covalent bonds between its two helium atoms, each of which contains two electrons. this gives it a total of four electrons, resulting in a bond order of 2.

Solved Consider The Following Two Molecules Draw Both Chegg Consider the following molecules, h2, he2, and o2. a) which one has a bond order of 2? b) which one has a single bond? c) which is the most unstable? d) write them in order of increasing stability. thank you!. For the following questions, choose all the answers that are correct: which of the following molecules are capable of hydrogen bonding with themselves?. Question: 1) consider the following two molecules, methane (ch4, left ) and chloroform (chcl3, right). vsepr tells us that both molecules have a tetrahedral shape. Question 1. consider the following two molecules: a) draw out all significant resonance structures for each molecule. (4 points) b) which molecule do you expect to be a better electrophile, and why?.
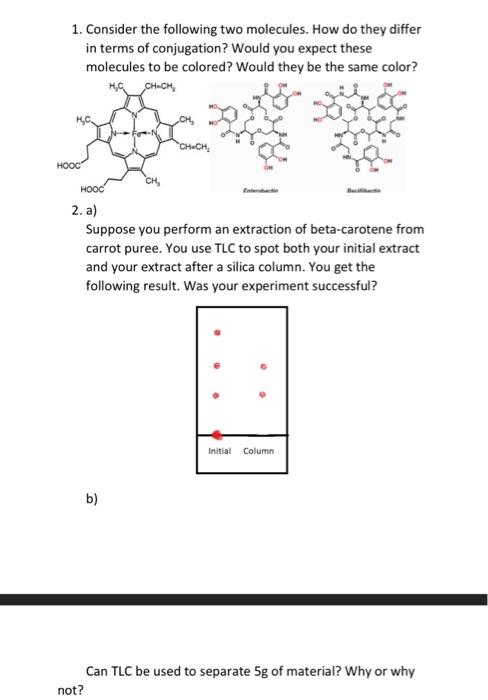
Solved 1 Consider The Following Two Molecules How Do They Chegg Question: 1) consider the following two molecules, methane (ch4, left ) and chloroform (chcl3, right). vsepr tells us that both molecules have a tetrahedral shape. Question 1. consider the following two molecules: a) draw out all significant resonance structures for each molecule. (4 points) b) which molecule do you expect to be a better electrophile, and why?. Answer question 1 all the three methods are good to distinguish between two molecules but the simplest and best method to observe the presence of carbonyl grpup is ir spectroscopy and nmr. Study with quizlet and memorize flashcards containing terms like what is the relationship between the following two molecules?, a compound contains hydroxyl groups as its predominant functional group. Consider the following molecules, h2, he2, and o2. a) which one has a bond order of 2? b) which one has a single bond? c) which is the most unstable? d) write them in order of increasing stability. A molecule is polar if it has a significant difference in electronegativity between its atoms, leading to an uneven distribution of charge. nonpolar molecules, on the other hand, have an even distribution of charge, often due to symmetrical arrangements of identical atoms or balanced dipoles.
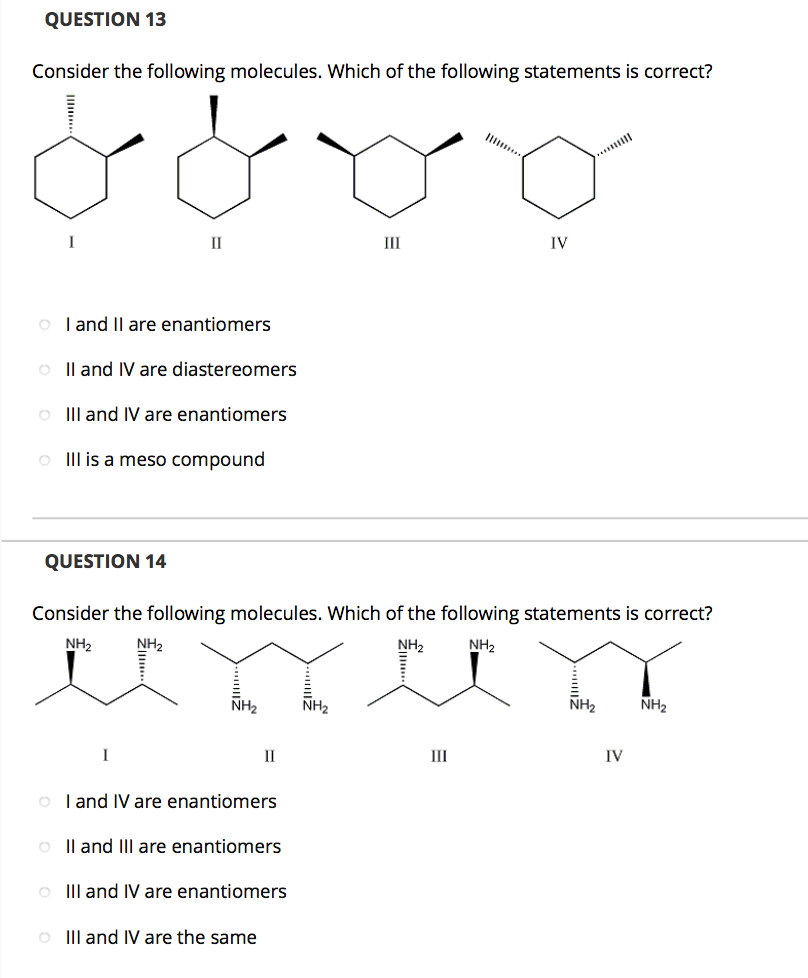
Solved Consider The Following Molecules Which Of The Chegg Answer question 1 all the three methods are good to distinguish between two molecules but the simplest and best method to observe the presence of carbonyl grpup is ir spectroscopy and nmr. Study with quizlet and memorize flashcards containing terms like what is the relationship between the following two molecules?, a compound contains hydroxyl groups as its predominant functional group. Consider the following molecules, h2, he2, and o2. a) which one has a bond order of 2? b) which one has a single bond? c) which is the most unstable? d) write them in order of increasing stability. A molecule is polar if it has a significant difference in electronegativity between its atoms, leading to an uneven distribution of charge. nonpolar molecules, on the other hand, have an even distribution of charge, often due to symmetrical arrangements of identical atoms or balanced dipoles.
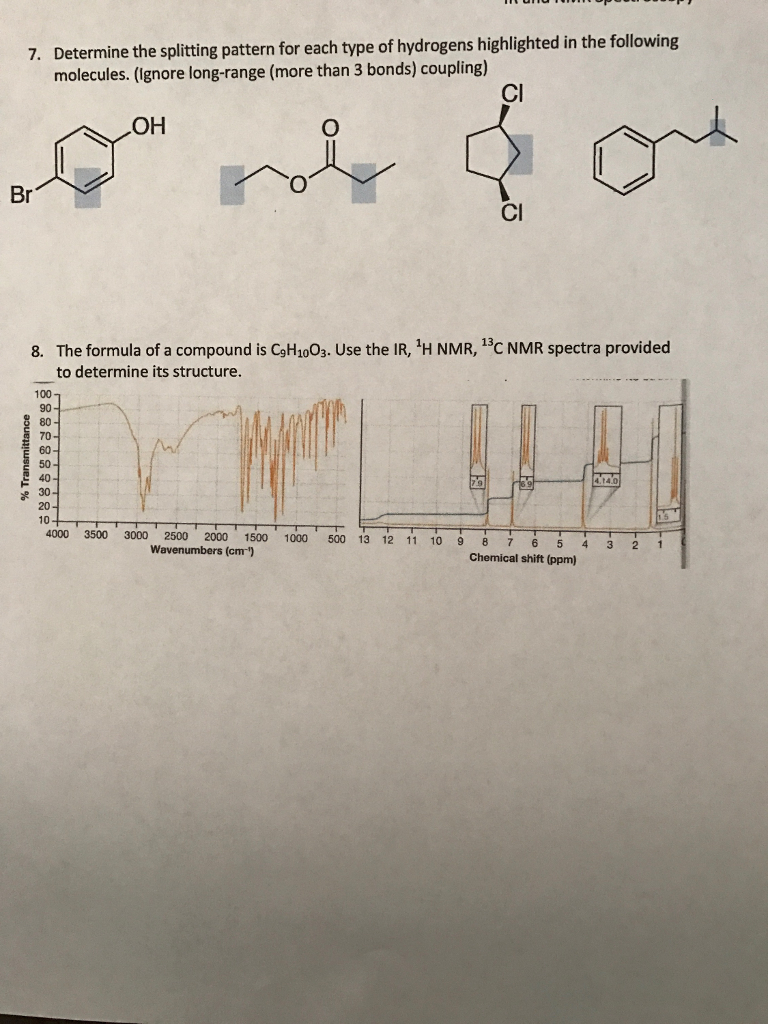
Solved 1 Consider The Following Two Molecules Which Would Chegg Consider the following molecules, h2, he2, and o2. a) which one has a bond order of 2? b) which one has a single bond? c) which is the most unstable? d) write them in order of increasing stability. A molecule is polar if it has a significant difference in electronegativity between its atoms, leading to an uneven distribution of charge. nonpolar molecules, on the other hand, have an even distribution of charge, often due to symmetrical arrangements of identical atoms or balanced dipoles.

Solved 3 Consider The Following Two Molecules A Draw The Chegg
Comments are closed.