Solved 11 Consider The Following Reaction 2 No G O2 G Chegg
Solved Consider The Following Reaction 2no G O 2 G Chegg Consider the following reversible reaction at equilibrium: 2 no (g) o2 (g) > 2 no2 (g). if the pressure inside the reaction vessel is increased, the equilibrium will shift to the right. For example, a negative Δg means that the reaction can occur without any external input of energy. this relates to common reactions, like combustion, where products are favored and released energy makes the process spontaneous.
Solved Consider The Following Reaction 2no G O 2 G Chegg Determine and for the reaction using standard enthalpy and entropy values from a data table. this video solution was recommended by our tutors as helpful for the problem above. was this helpful? here are the essential concepts you must grasp in order to answer the question correctly. Consider the following reaction at equilibrium: 2 no (g) o2 (g) ⇌ 2 no2 (g) in most cases, kc ≠ kp so the following equation is used to convert between them: consider the equilibrium and answer the questions below. round each answer to two places past the decimal in scientific notation. Consider the reaction between no (g)and o2 (g)represented below. what is the balanced equation for this reaction and what is the limiting reactant?. The rate law consistent with the reaction mechanism is rate = k[no]2[o2], where the reaction is second order in no and first order in o2. this is derived from considering the rate determining step and substituting the intermediate concentration into the rate equation.

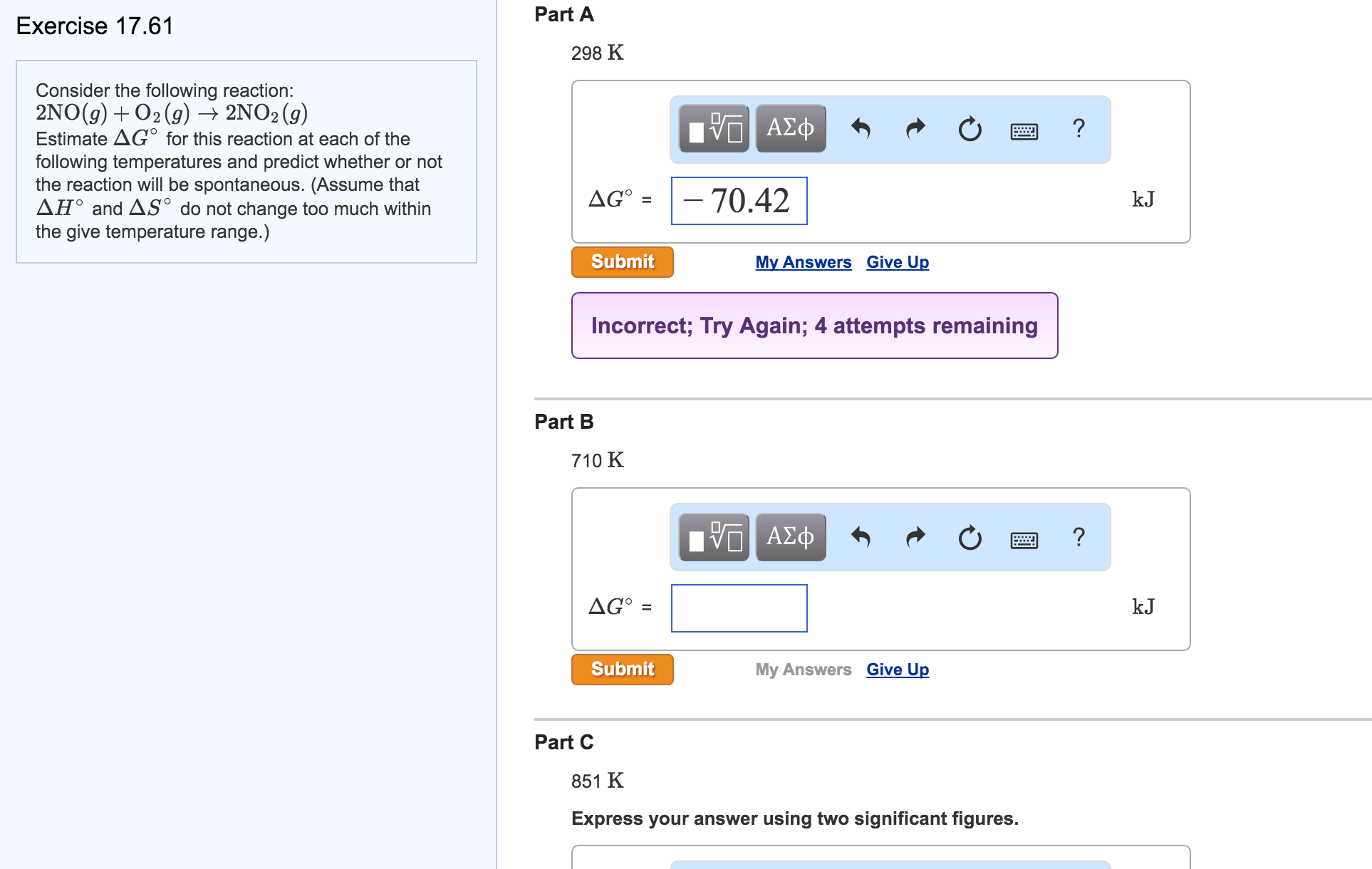
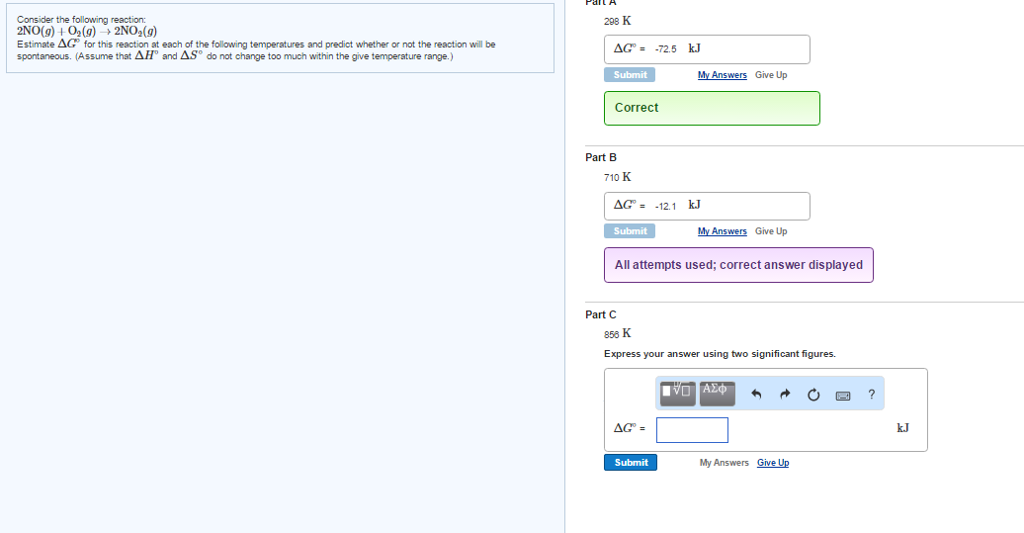
Solved 11 Consider The Following Reaction 2 No G O2 G Chegg Consider the reaction between no (g)and o2 (g)represented below. what is the balanced equation for this reaction and what is the limiting reactant?. The rate law consistent with the reaction mechanism is rate = k[no]2[o2], where the reaction is second order in no and first order in o2. this is derived from considering the rate determining step and substituting the intermediate concentration into the rate equation. Consider the reaction: 2 no (g) o2 (g) → 2 no2 (g). estimate Δg° for this reaction at each temperature and predict whether or not the reaction is spontaneous. Consider the reaction: 2 no (g) o2(g) ⇌ 2 no2(g) the following data show the equilibrium constant for this reaction measured at several different temperatures. use the data to find Δh°rxn and Δs°rxn for the reaction.
Comments are closed.