Solved 11 Radioactive Decay Follows First Order Kinetics Chegg

Solved Radioactive Decay Follows Zero Order Kinetics First Chegg Radioactive decay follows a first order kinetics. a radioactive element takes 11 min to decay 27% of its nuclei, calculate its half life in seconds. give your answer with at least two decimals. (note: these values are not realistic and nuclear decay is typically a much slower process) here’s the best way to solve it. Problem #8: a radioactive isotope has a half life of 1.2 billion years. as measured by the presence of the isotope and its stable decay product, a rock originally contained 10 grams of the radioactive isotope, and now contains 1.25 grams.
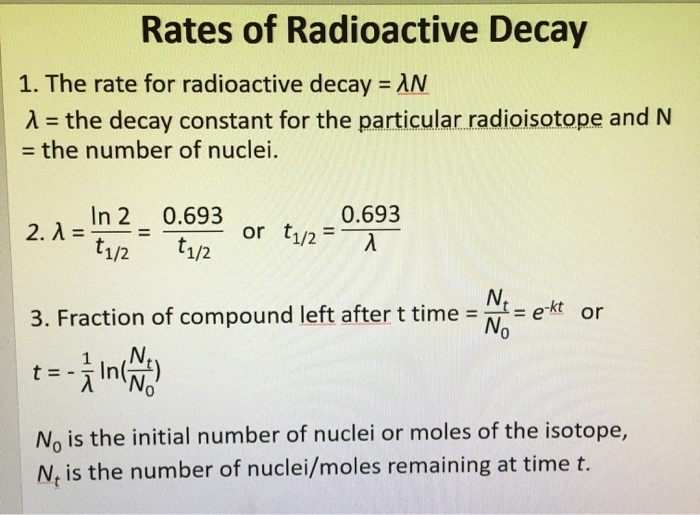
Solved Radioactive Decay And Kinetics Radioactive Decay Chegg Because nuclear decay reactions follow first order kinetics and have a rate constant that is independent of temperature and the chemical or physical environment, we can perform similar calculations using the half lives of isotopes to estimate the ages of geological and archaeological artifacts. Radioactive decay follows first order kinetics. the initial amount of two radioactive elements x and y is 1 gm each. what will be the ratio of x and y after two days if their half lives are 12 hours and 16 hours respectively?. To solve the problem regarding the decay of radioactive elements and their activity over time, we will follow these steps: 1. understanding the concept of half life: the half life (t1 2) is the time required for half of the radioactive substance to decay. Radioactive decay follows order kinetics. a) first b) second c) third d) zero.
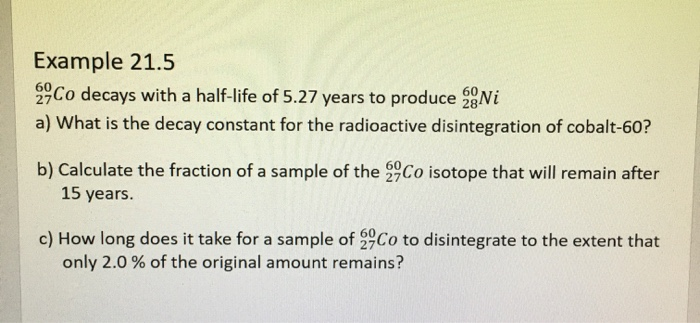
Solved Radioactive Decay And Kinetics Radioactive Decay Chegg To solve the problem regarding the decay of radioactive elements and their activity over time, we will follow these steps: 1. understanding the concept of half life: the half life (t1 2) is the time required for half of the radioactive substance to decay. Radioactive decay follows order kinetics. a) first b) second c) third d) zero. Find step by step chemistry solutions and your answer to the following textbook question: all radioactive decay processes follow first order kinetics. what does this mean?. The correct option is a solution and explanation radioactive decay follows first order kinetics. the rate of decay is proportional to the amount of the substance remaining, and its half life is independent of the initial concentration. thus, the correct answer is (a). Our expert help has broken down your problem into an easy to learn solution you can count on. question: radioactive decay follows a first order kinetics. a radioactive nucleus has a half life of 2.4 minutes. how many grams are left after a sample of 31.06 grams is left decomposing for 33.3 seconds? give your answer with at least two decimals. In the case of radioactive decay, the rate of decay is directly proportional to the number of radioactive atoms present. therefore, radioactive decay follows first order kinetics.
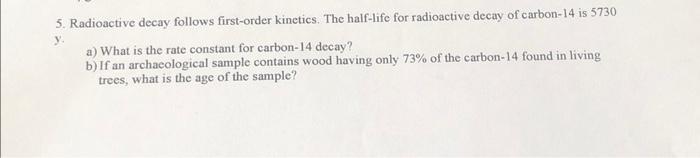
Solved 5 Radioactive Decay Follows First Order Kinetics Chegg Find step by step chemistry solutions and your answer to the following textbook question: all radioactive decay processes follow first order kinetics. what does this mean?. The correct option is a solution and explanation radioactive decay follows first order kinetics. the rate of decay is proportional to the amount of the substance remaining, and its half life is independent of the initial concentration. thus, the correct answer is (a). Our expert help has broken down your problem into an easy to learn solution you can count on. question: radioactive decay follows a first order kinetics. a radioactive nucleus has a half life of 2.4 minutes. how many grams are left after a sample of 31.06 grams is left decomposing for 33.3 seconds? give your answer with at least two decimals. In the case of radioactive decay, the rate of decay is directly proportional to the number of radioactive atoms present. therefore, radioactive decay follows first order kinetics.
Comments are closed.