Solved 18 Given The Following Reactions N2 G O2 Chegg

Solved 18 Given The Following Reactions N2 G O2 Chegg Question: 18) given the following reactions n2 ( g) o2 ( g)−2no (g)Δh= 180.7 kj 2no (g) o2 ( g)−2no2 ( g)Δh=−113.1 kj the enthalpy of reaction for 4no (g)−2no2 ( g) n2 ( g) is kj. To find the enthalpy of the reaction 2n2o (g) → 2no (g) n2 (g), we can use hess's law which states that the total enthalpy change in a reaction is independent of the pathway.

Solved 12 Given The Following Reactions N2 G O2 G Chegg Consider the following natural language sentence: uli brought wine to the party, but i brought nothing because i forgot. which answer is a translation of this natural language sentence into formal logic?. Given the following reactions n2 (g) o2 (g) → 2no (g) Δh = 180.7 kj 2n2o (g) → o2 (g) 2n2 (g) Δh = answered step by step solved by verified expert eastern new mexico university. N2 (g) o2 (g) → 2no (g), we can use hess's law, which states that the total enthalpy change for a reaction is the sum of the enthalpy changes for the individual steps. Calculate the heat (kj) released to the surroundings when 38.5 g of o2 (g) reacts with excess co. the specific heat of liquid bromine (br2) is 0.226 j g k. how much heat (j) is required to raise the temperature of 10.0 ml of bromine from 25.00 °c to 27.30 °c? the density of liquid bromine: 3.12 g ml.

Solved 18 Given The Following Reactions N2 G O2 Chegg N2 (g) o2 (g) → 2no (g), we can use hess's law, which states that the total enthalpy change for a reaction is the sum of the enthalpy changes for the individual steps. Calculate the heat (kj) released to the surroundings when 38.5 g of o2 (g) reacts with excess co. the specific heat of liquid bromine (br2) is 0.226 j g k. how much heat (j) is required to raise the temperature of 10.0 ml of bromine from 25.00 °c to 27.30 °c? the density of liquid bromine: 3.12 g ml. Video answer: to calculate delta h for the reaction of interest, we need to see how to combine these 2 reactions with known delta h values in order to get the overall reaction. Temperature plays a crucial role in determining the spontaneity of a reaction as it affects the balance between enthalpy and entropy. in the gibbs free energy equation, increasing temperature (t) can enhance the impact of entropy (Δs) on Δg. Given the following reactions: n2 (g) o2 (g) → 2 no (g) Δh°rxn= 180.7 kj 2 no (g) o2→ 2 no2 (g) Δh°rxn= 113.1 kj determine Δh for the following reaction: 4 no (g) →2 no2 (g) n2 (g). a. 45.5 kj b. 293.8 kj c. 45.5 kj d. 293.8 kj e. 67.6 kj f. 67.6 kj. your solution’s ready to go!. We first need to reverse the third reaction and double the second reaction to get n2o (g) no2 (g) on the reactant side of the equation. the enthalpy change for this will be the sum of the enthalpy changes for these manipulated reactions, using hess's law.

Solved 10 Given The Following Reactions N G O2 G Chegg Video answer: to calculate delta h for the reaction of interest, we need to see how to combine these 2 reactions with known delta h values in order to get the overall reaction. Temperature plays a crucial role in determining the spontaneity of a reaction as it affects the balance between enthalpy and entropy. in the gibbs free energy equation, increasing temperature (t) can enhance the impact of entropy (Δs) on Δg. Given the following reactions: n2 (g) o2 (g) → 2 no (g) Δh°rxn= 180.7 kj 2 no (g) o2→ 2 no2 (g) Δh°rxn= 113.1 kj determine Δh for the following reaction: 4 no (g) →2 no2 (g) n2 (g). a. 45.5 kj b. 293.8 kj c. 45.5 kj d. 293.8 kj e. 67.6 kj f. 67.6 kj. your solution’s ready to go!. We first need to reverse the third reaction and double the second reaction to get n2o (g) no2 (g) on the reactant side of the equation. the enthalpy change for this will be the sum of the enthalpy changes for these manipulated reactions, using hess's law.
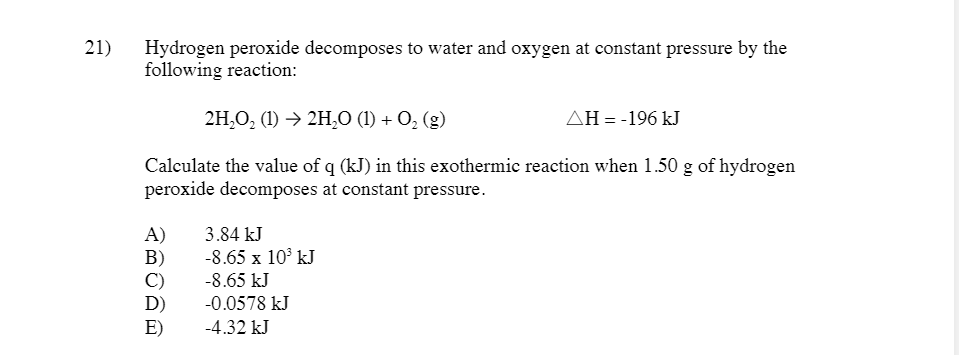
Solved 10 Given The Following Reactions N G O2 G Chegg Given the following reactions: n2 (g) o2 (g) → 2 no (g) Δh°rxn= 180.7 kj 2 no (g) o2→ 2 no2 (g) Δh°rxn= 113.1 kj determine Δh for the following reaction: 4 no (g) →2 no2 (g) n2 (g). a. 45.5 kj b. 293.8 kj c. 45.5 kj d. 293.8 kj e. 67.6 kj f. 67.6 kj. your solution’s ready to go!. We first need to reverse the third reaction and double the second reaction to get n2o (g) no2 (g) on the reactant side of the equation. the enthalpy change for this will be the sum of the enthalpy changes for these manipulated reactions, using hess's law.
Comments are closed.