Solved 2 Consider Three Sparingly Soluble Salts Agcl Chegg

Solved 2 Consider Three Sparingly Soluble Salts Agcl Chegg Chemistry questions and answers 2. consider three sparingly soluble salts: agcl compound pbf2 pbf2 baco does solubility in aqueous solution change with ph? baco complete the table and select all compounds whose solubility in aqueous solution changes with pl. a) agci, pbf2 and baco3 b) agci and pbf2 c) agcl and baco3 d) pbf2 and baco3 b agci only. Step 1 2first, we need to determine whether the solubility of the three sparingly soluble salts changes with ph. generally, the solubility of sparingly soluble salts is not affected by ph, unless they contain acidic or basic functional groups that can undergo protonation or deprotonation reactions.
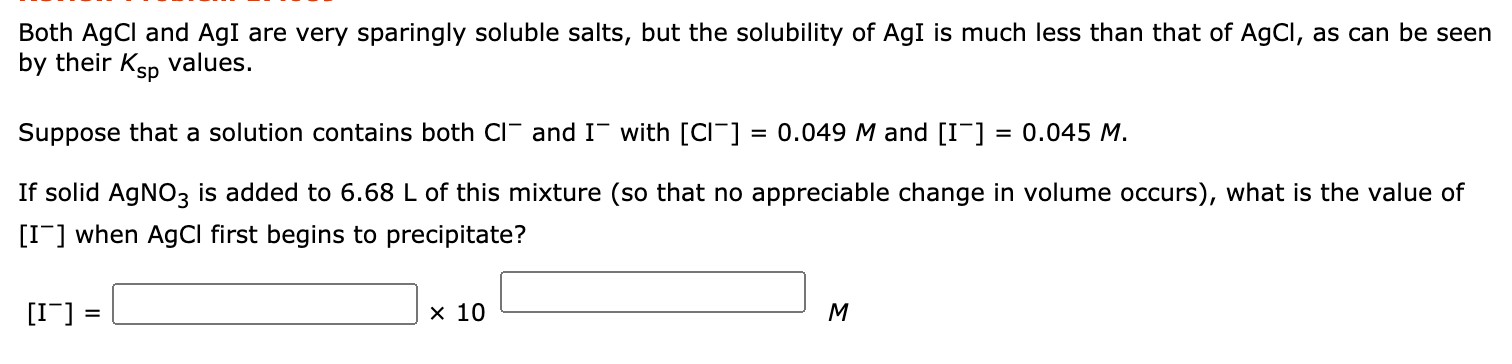
Solved Both Agcl And Agi Are Very Sparingly Soluble Salts Chegg According to le chatelier's principle, the addition of ag ions from agno 3 to the solution of agcl shifts the solubility equilibrium of agcl from right to left. Ram 2 days ago both agcl and agi are sparingly soluble salts (ksp agcl = 1.8 x 10 10, ksp agi = 8.3 x 10 17). suppose a solution contains both [cl ] = 0.050 m and [i ] = 0.050 m. if solid agno3 is added to 1.00 l of this solution (you can assume that the volume remains constant), what is the value of [i ] when agcl first begins to precipitate?. Explanation: ammonia (nh3) can form a complex with ag ions (ag (nh3)2 ), which effectively removes ag from the solution and shifts the equilibrium to the right, increasing the solubility of agcl. A. solubility of agcl is more in aqueous agno3 solution than in pure water: this statement is incorrect because the presence of ag ions from agno3 will decrease the solubility of agcl due to the common ion effect.
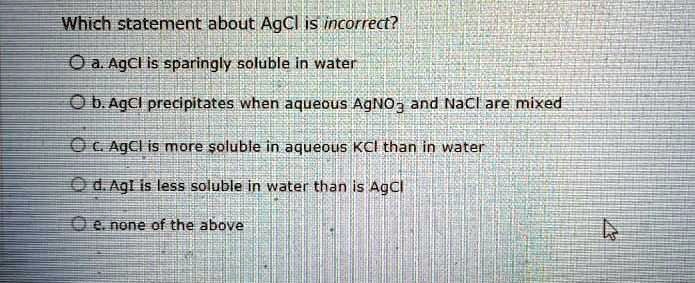
Which Statement About Agcl Is Incorrect Oa Agcl Is Sparingly Soluble In Explanation: ammonia (nh3) can form a complex with ag ions (ag (nh3)2 ), which effectively removes ag from the solution and shifts the equilibrium to the right, increasing the solubility of agcl. A. solubility of agcl is more in aqueous agno3 solution than in pure water: this statement is incorrect because the presence of ag ions from agno3 will decrease the solubility of agcl due to the common ion effect. Figure 1 silver chloride is a sparingly soluble ionic solid. when it is added to water, it dissolves slightly and produces a mixture consisting of a very dilute solution of ag and cl– ions in equilibrium with undissolved silver chloride. source: openstax chemistry 2e. The three common silver halides ( agcl, agbr, and agi ) are all sparingly soluble salts. given the values for ksp for these salts below, calculate the concentration of silver ion, in mol l, in a ksp. When a sparingly soluble ionic solid, such as agcl, dissolves in water, ions break away from the crystal lattice and enter into the solution and become hydrated. hydrated ions can also lose their “waters of hydration” ag (aq) and add back onto the surface of the crystal. The three common silver halides (agcl, agbr, and agi) are all sparingly soluble salts.
Comments are closed.