Solved 2 The Changes In Enthalpy And Entropy Are Derived As Chegg

Solved 2 The Changes In Enthalpy And Entropy Are Derived As Chegg The changes in enthalpy and entropy are derived as functions of pressure and temperature in the textbook. ideal gas with a constant heat capacity, cp= (7 2)r, undergoes a mechanically reversible process from 350∘c and 5 atm, to 1 atm by different paths. Roughly speaking, the energy changes that we looked at in the introduction to thermodynamics were changes in enthalpy. we will see in the next section that there is another energetic factor, entropy, that we also need to consider in reactions.

Solved 3 Entropy And Enthalpy Changes Calculate The Chegg The change in enthalpy and entropy are derived as functions of pressure and temperature in the textbook. ideal gas with constant heat capacity, cp= (7 2)r, undergoes a mechanically reversible process from 350c and 5atm, to 1 atm by different paths. Now, let's derive enthalpy and entropy changes as functions of t and p. the starting equations are as follows. dh = dt cp), dp as ds = t dp t derive the final equations using the definition of cand maxwell relations. 2 15. To analyze what this really means, let’s use the fact that changes in entropy are exact and we can examine adiabatic transitions using two separate steps as shown in figure 4.2. under adiabatic conditions both the volume and temperature of the system are affected. Entropy is a measure of the disorder of a system. entropy also describes how much energy is not available to do work. the more disordered a system and higher the entropy, the less of a system's energy is available to do work. the meaning of entropy is difficult to grasp, as it may seem like an abstract concept.
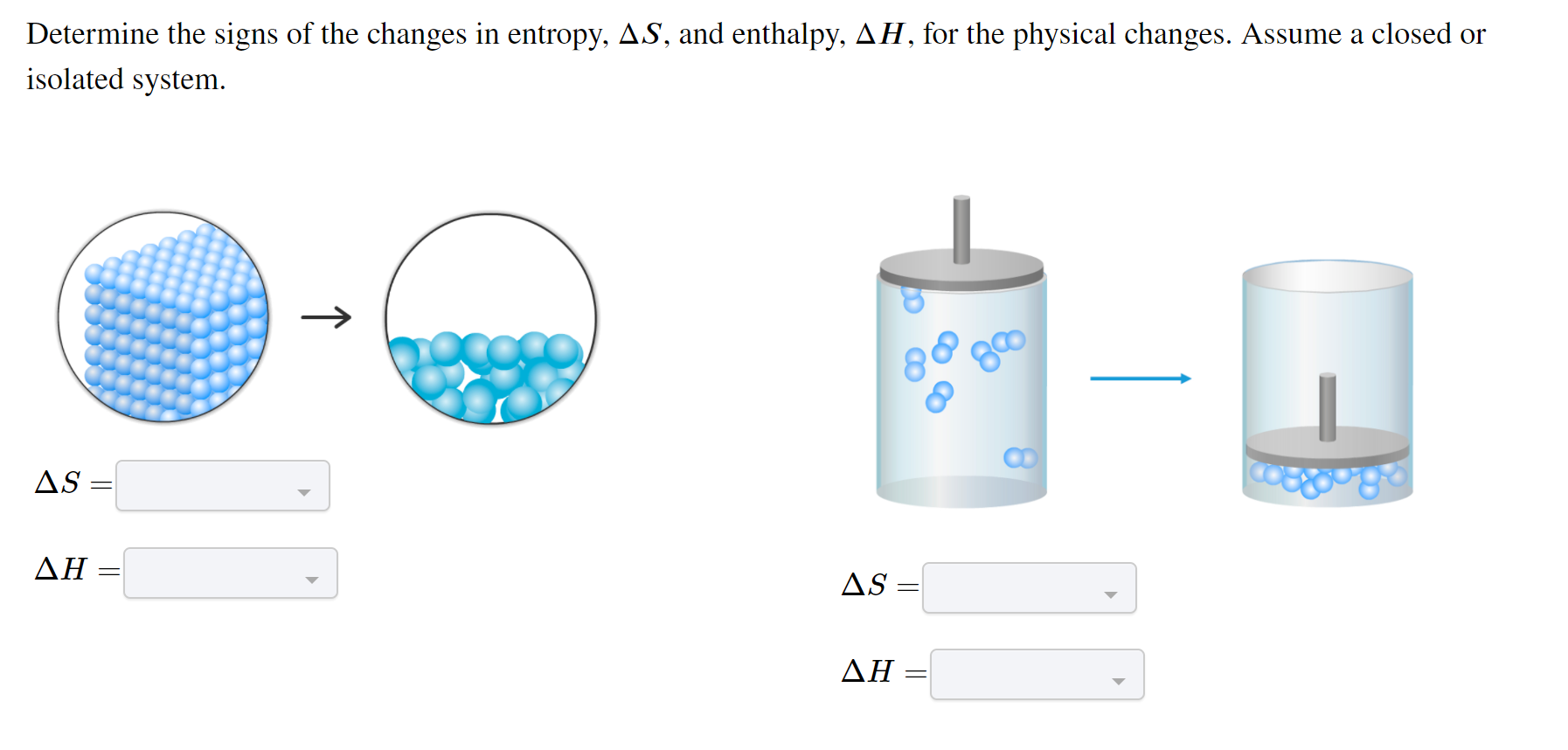
Solved Determine The Signs Of The Changes In Entropy As Chegg To analyze what this really means, let’s use the fact that changes in entropy are exact and we can examine adiabatic transitions using two separate steps as shown in figure 4.2. under adiabatic conditions both the volume and temperature of the system are affected. Entropy is a measure of the disorder of a system. entropy also describes how much energy is not available to do work. the more disordered a system and higher the entropy, the less of a system's energy is available to do work. the meaning of entropy is difficult to grasp, as it may seem like an abstract concept. Substances act as reservoirs of energy, meaning that energy can be added to them or removed from them. energy is stored in a substance when the kinetic energy of its atoms or molecules is raised. You will not be encountering ideal gases often, so let us compare an ideal gas calculation with a 'real' gas, familiar calculation of heat, work, change in enthalpy, change in internal energy and change in entropy are required. Entropy changes are fairly easy to calculate so long as one knows initial and final state. for example, if the initial and final volume are the same, the entropy can be calculated by assuming a …. The reversible heat is the enthalpy change for the transition, and the entropy change is the enthalpy change divided by the thermodynamic temperature. [59] for fusion (i.e., melting) of a solid to a liquid at the melting point , the entropy of fusion is: similarly, for vaporisation of a liquid to a gas at the boiling point , the entropy of.
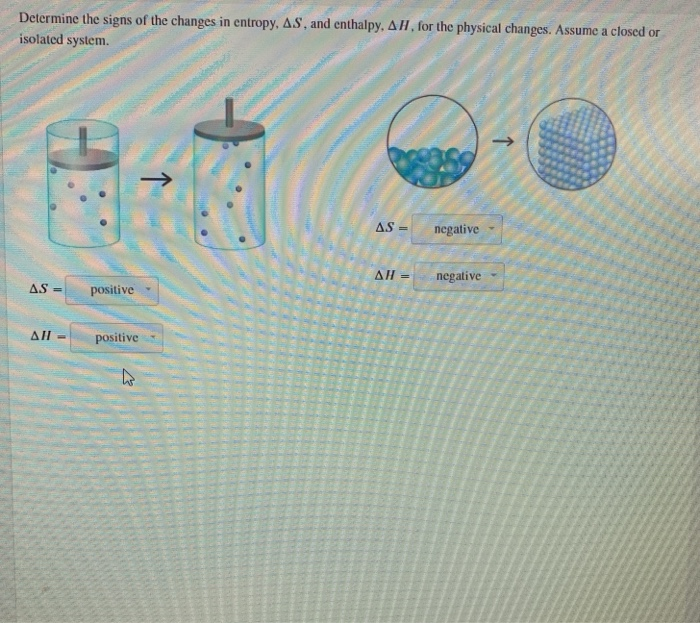
Solved Determine The Signs Of The Changes In Entropy As Chegg Substances act as reservoirs of energy, meaning that energy can be added to them or removed from them. energy is stored in a substance when the kinetic energy of its atoms or molecules is raised. You will not be encountering ideal gases often, so let us compare an ideal gas calculation with a 'real' gas, familiar calculation of heat, work, change in enthalpy, change in internal energy and change in entropy are required. Entropy changes are fairly easy to calculate so long as one knows initial and final state. for example, if the initial and final volume are the same, the entropy can be calculated by assuming a …. The reversible heat is the enthalpy change for the transition, and the entropy change is the enthalpy change divided by the thermodynamic temperature. [59] for fusion (i.e., melting) of a solid to a liquid at the melting point , the entropy of fusion is: similarly, for vaporisation of a liquid to a gas at the boiling point , the entropy of.
Solved 3 Enthalpy And Entropy Changes A What Is The Chegg Entropy changes are fairly easy to calculate so long as one knows initial and final state. for example, if the initial and final volume are the same, the entropy can be calculated by assuming a …. The reversible heat is the enthalpy change for the transition, and the entropy change is the enthalpy change divided by the thermodynamic temperature. [59] for fusion (i.e., melting) of a solid to a liquid at the melting point , the entropy of fusion is: similarly, for vaporisation of a liquid to a gas at the boiling point , the entropy of.
Comments are closed.