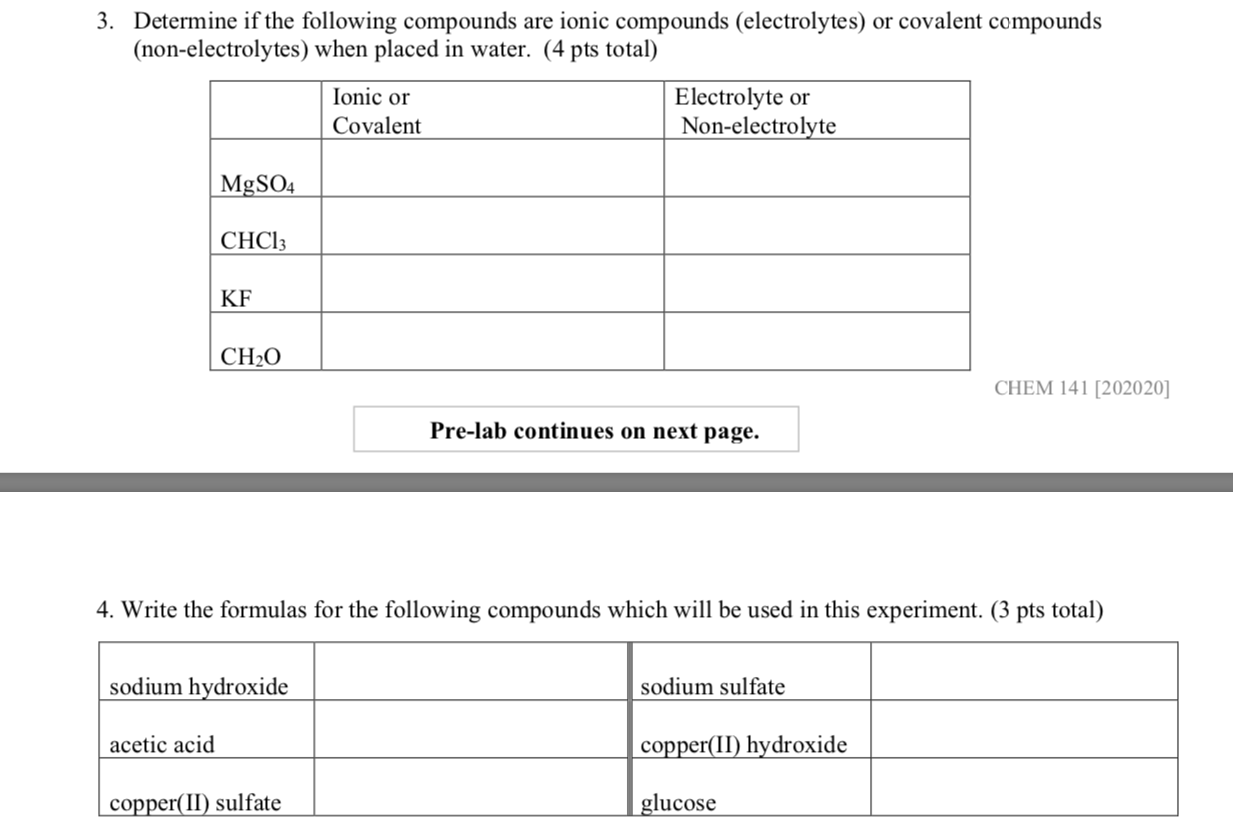
Solved 3 Determine If The Following Compounds Are Ionic Chegg Write the formulas for the following compounds which will be used in this experiment. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. question: 3. Determine whether each of the following compounds would be best represented by an ionic or a covalent lewis structure. use lewis theory to determine the formula for the compound that forms from mg and br.
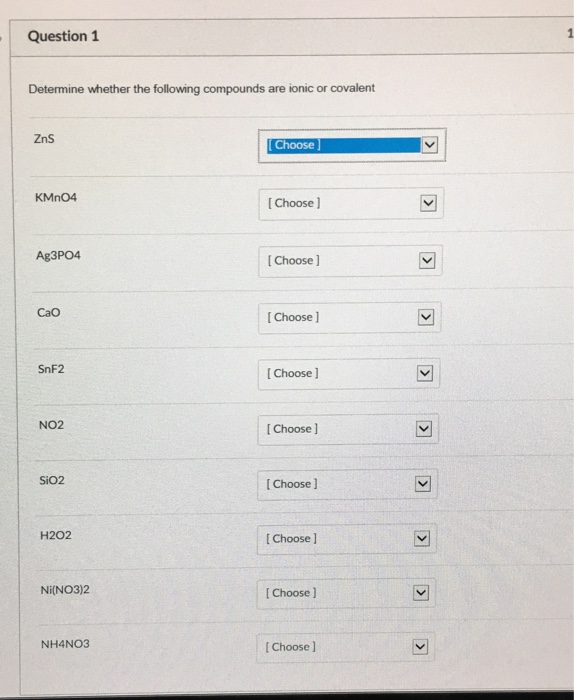
Solved Determine Whether The Following Compounds Are Ionic Chegg Ionic ionic compounds are named by first identifying the positive ion (the cation) and then the negative ion (the anion). you should note that the cation is always the metal and the anion the nonmetal. the positive ion takes the same name as it's element, the negative ion takes the first part of its element name plus an ide ending. Determine if the elements in the following compounds are metals or nonmetals. describe the type of boding that occurs in the compound. compound element 1 element 2 bond type. how are ionic bonds and covalent bonds different? how does a polar covalent bond differ from a nonpolar covalent bond?. Ionic bond: a bond formed between metal and non metal is called ionic bond molecular bond: a bond formed bet …view the full answer. Determine whether the following pairs of elements can form ionic compounds. we have an expert written solution to this problem! what is the formula for an ionic compound that contains the elements calcium and fluorine? classify the substances as atomic elements, molecular elements, molecular compounds, or ionic compounds.

Solved Name The Following Ionic Compounds Chegg Ionic bond: a bond formed between metal and non metal is called ionic bond molecular bond: a bond formed bet …view the full answer. Determine whether the following pairs of elements can form ionic compounds. we have an expert written solution to this problem! what is the formula for an ionic compound that contains the elements calcium and fluorine? classify the substances as atomic elements, molecular elements, molecular compounds, or ionic compounds. Our expert help has broken down your problem into an easy to learn solution you can count on. question: 3. (6 points) determine if the following ionic compounds are soluble or insoluble. explain your reasoning for each. (bt3: apply) a. fes b. ag2so4 4. (6 points) explain how you would identify the following types of reactions. (bt2: understand) a. 1) predict whether the bonds in the following species are ionic or covalent. 5) use the group number to determine the charge on an ion derived from each element. 4) how many protons and electrons are present in each ion?. Using the periodic table, predict whether the following chlorides are ionic or covalent: sicl 4, pcl 3, cacl 2, cscl, cucl 2, and crcl 3. for each of the following compounds, state whether it is ionic or covalent. if it is ionic, write the symbols for the ions involved:. To determine whether each compound is molecular or ionic, we can analyze their components based on the types of elements involved and their bonding characteristics. ti₂s₃: this compound consists of titanium (a metal) and sulfur (a nonmetal). typically, when a metal bonds with a nonmetal, it forms an ionic compound. so, ti₂s₃ is ionic.
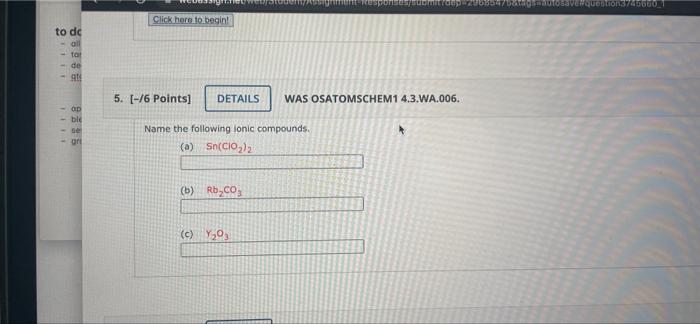
Solved Name The Following Ionic Compounds Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. question: 3. (6 points) determine if the following ionic compounds are soluble or insoluble. explain your reasoning for each. (bt3: apply) a. fes b. ag2so4 4. (6 points) explain how you would identify the following types of reactions. (bt2: understand) a. 1) predict whether the bonds in the following species are ionic or covalent. 5) use the group number to determine the charge on an ion derived from each element. 4) how many protons and electrons are present in each ion?. Using the periodic table, predict whether the following chlorides are ionic or covalent: sicl 4, pcl 3, cacl 2, cscl, cucl 2, and crcl 3. for each of the following compounds, state whether it is ionic or covalent. if it is ionic, write the symbols for the ions involved:. To determine whether each compound is molecular or ionic, we can analyze their components based on the types of elements involved and their bonding characteristics. ti₂s₃: this compound consists of titanium (a metal) and sulfur (a nonmetal). typically, when a metal bonds with a nonmetal, it forms an ionic compound. so, ti₂s₃ is ionic.

Solved Question 3for Each Of The Following Compounds State Chegg Using the periodic table, predict whether the following chlorides are ionic or covalent: sicl 4, pcl 3, cacl 2, cscl, cucl 2, and crcl 3. for each of the following compounds, state whether it is ionic or covalent. if it is ionic, write the symbols for the ions involved:. To determine whether each compound is molecular or ionic, we can analyze their components based on the types of elements involved and their bonding characteristics. ti₂s₃: this compound consists of titanium (a metal) and sulfur (a nonmetal). typically, when a metal bonds with a nonmetal, it forms an ionic compound. so, ti₂s₃ is ionic.

Solved State Whether The Following Compounds Are Ionic Or Chegg
