
Solved 42 Balance The Following Equation 4pts Chegg Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. there are 2 steps to solve this one. not the question you’re looking for? post any question and get expert help quickly. Any students who submit the same set up and work (i.e., copy) will receive zero points on this assignment. 1) (4 pts) what are the two definitions of equilibrium? 2) (4 pts) write equilibrium constant expressions, k., for the following balanced.
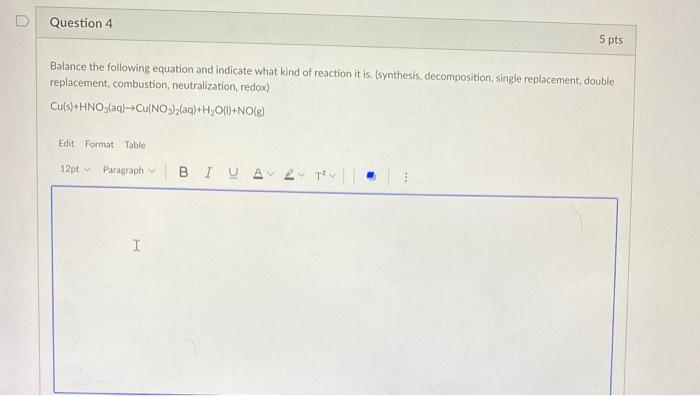
Solved Question 4 5 Pts Balance The Following Equation And Chegg Use the calculator below to balance chemical equations and determine the type of reaction (instructions). to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. the balanced equation will appear above. use uppercase for the first character in the element and lowercase for the second character. Enter an equation of a chemical reaction and click 'balance'. the answer will appear below. always use the upper case for the first character in the element name and the lower case for the second character. examples: fe, au, co, br, c, o, n, f. compare: co cobalt and co carbon monoxide. (7.1) balance each of the following chemical equations: 1. mg(s) agno3(aq) → (no3)2(aq) ag(s) 2. al(s) cuso4(aq) → al2(so4)3(aq) cu(s) 3. pb(no3)2(aq) nacl(aq) → pbcl2(s) nano3(aq) 4. hcl(aq) al(s) → h2(g) alcl3(aq). For each of the following reactions, balance the chemical equation ( 4pts total) a) ba (oh)2 hcl→bacl2 h2o c) pb …nacl→ [pbcl2 na d) fe o2→≃fe2o3. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. question: 3.
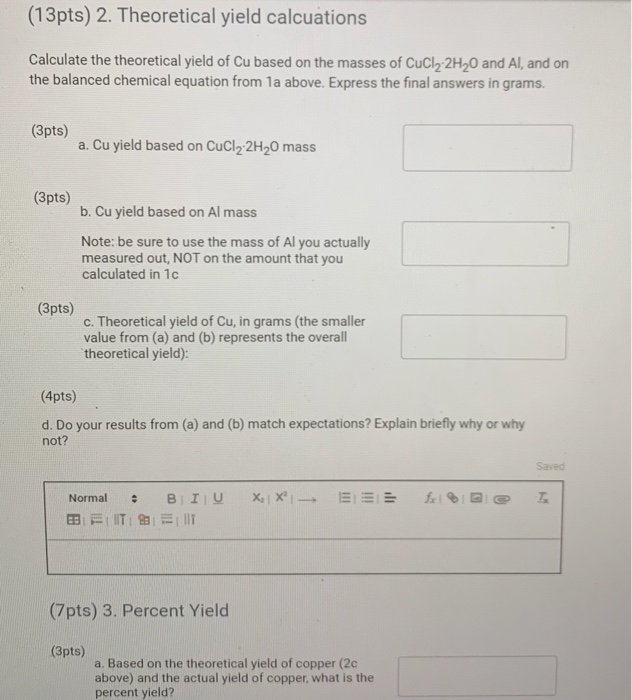
Solved 10pts 1 Calculations 4pts A What Is The Chegg (7.1) balance each of the following chemical equations: 1. mg(s) agno3(aq) → (no3)2(aq) ag(s) 2. al(s) cuso4(aq) → al2(so4)3(aq) cu(s) 3. pb(no3)2(aq) nacl(aq) → pbcl2(s) nano3(aq) 4. hcl(aq) al(s) → h2(g) alcl3(aq). For each of the following reactions, balance the chemical equation ( 4pts total) a) ba (oh)2 hcl→bacl2 h2o c) pb …nacl→ [pbcl2 na d) fe o2→≃fe2o3. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. question: 3. Study with quizlet and memorize flashcards containing terms like which coefficient placed before hcl will balance the following equation? zn hcl→zncl2 h2, in the expression 3h2o, the 3 is a , while the 2 is a ., fill in the missing coefficients to balance the chemical equation below. Write a balanced chemical equation based on the following description: solid chromium reacts with solid iodine to form solid chromium (iii) iodide. we have an expert written solution to this problem! complete the balanced molecular chemical equation for the reaction below. if no reaction occurs, write nr after the reaction arrow. Balancing chemical equations involves ensuring that the number of atoms of each element is equal on both sides of the equation. this process requires systematic adjustments to the coefficients of the reactants and products until the conservation of mass is achieved. Answer to solved 3. for each of the following reactions, balance the | chegg.

Solved Question 13balance The Following Equation What Will Chegg Study with quizlet and memorize flashcards containing terms like which coefficient placed before hcl will balance the following equation? zn hcl→zncl2 h2, in the expression 3h2o, the 3 is a , while the 2 is a ., fill in the missing coefficients to balance the chemical equation below. Write a balanced chemical equation based on the following description: solid chromium reacts with solid iodine to form solid chromium (iii) iodide. we have an expert written solution to this problem! complete the balanced molecular chemical equation for the reaction below. if no reaction occurs, write nr after the reaction arrow. Balancing chemical equations involves ensuring that the number of atoms of each element is equal on both sides of the equation. this process requires systematic adjustments to the coefficients of the reactants and products until the conservation of mass is achieved. Answer to solved 3. for each of the following reactions, balance the | chegg.
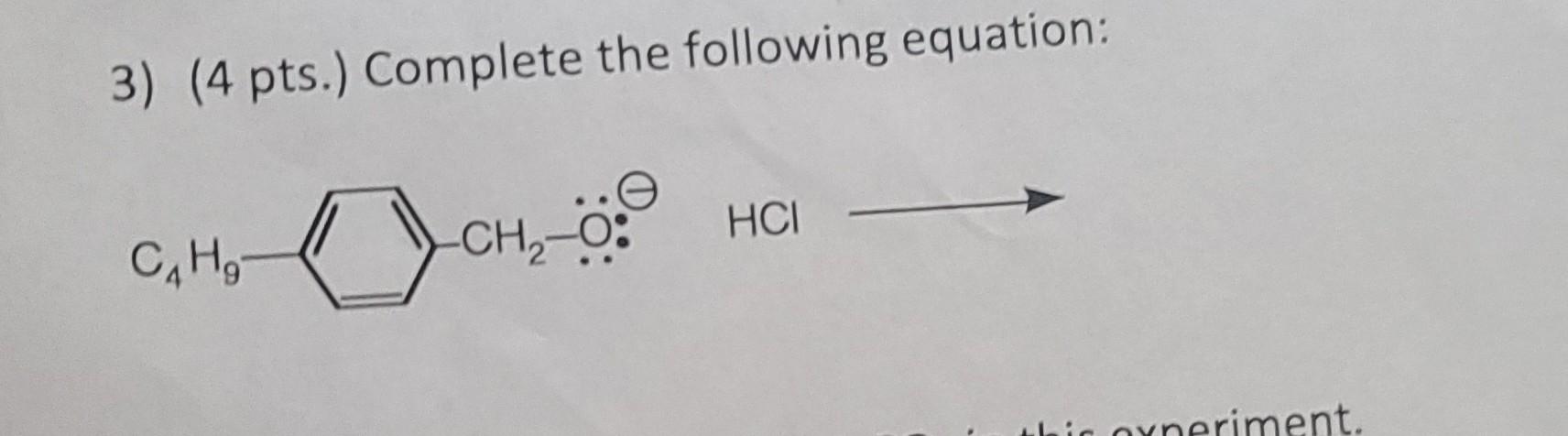
Solved 3 4 Pts Complete The Following Equation Chegg Balancing chemical equations involves ensuring that the number of atoms of each element is equal on both sides of the equation. this process requires systematic adjustments to the coefficients of the reactants and products until the conservation of mass is achieved. Answer to solved 3. for each of the following reactions, balance the | chegg.

Solved A B C 3 4 Pts Complete The Following Equation Chegg
