
Solved Balance The Following Equation Chegg Balance the following equation: (4pts) ca3p2 h2o→ph3 ca(oh)2 your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. There are 2 steps to solve this one. a. (h 2 so 4 (a q) koh (a q) → k 2 so 4 (a q) h 2 o (l)) 1. sulfur atoms are even by nature. not the question you’re looking for? post any question and get expert help quickly.

Solved 42 Balance The Following Equation 4pts Chegg Theoretical yield calcuations calculate the theoretical yield of cu based on the masses of cucl2 2h20 and al, and on the balanced chemical equation from 1a above. express the final answers in grams. (3pts) a. (4 pts.) balance the following chemical equation: ch4 o2 → co2 h2o how many grams of co2 are produced from 117 g of o2 and excess cha? the molar mass are as follows: o2 = 32.00 g mol, co2= 44.01 g mol. for full credit, you will need to show your work and calculations neatly and clearly. For each of the following reactions, balance the chemical equation ( 4pts total) a) ba (oh)2 hcl→bacl2 h2o c) pb …nacl→ [pbcl2 na d) fe o2→≃fe2o3. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. question: 3. (4 pts) for the balanced equation: 2co2(g) h2o(g) = 202(g) chco(g), calculate the concentration of oxygen at equilibrium at 150°c given: k. = 6.31 when [co2]oq=0.54 m, [hz0];, = 0.33 m, and (ch2co].q=0.92 m. c. (2 pts) what is for the equation 202(g) ch2co(g) = 2c02(g) h2o(g) at 150°c?.

Solved Question 13balance The Following Equation What Will Chegg For each of the following reactions, balance the chemical equation ( 4pts total) a) ba (oh)2 hcl→bacl2 h2o c) pb …nacl→ [pbcl2 na d) fe o2→≃fe2o3. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. question: 3. (4 pts) for the balanced equation: 2co2(g) h2o(g) = 202(g) chco(g), calculate the concentration of oxygen at equilibrium at 150°c given: k. = 6.31 when [co2]oq=0.54 m, [hz0];, = 0.33 m, and (ch2co].q=0.92 m. c. (2 pts) what is for the equation 202(g) ch2co(g) = 2c02(g) h2o(g) at 150°c?. Question: 27) (4pts) balance the following chemical equation. al(no3)3 nas nano3 als 28) (3pts) automotive air bags inflate when sodium azide decomposes to its constituent elements: 2nan3 (s) 2na (s) 3n2 (8) how many moles of n2 are produced by the decomposition of 1.75 mol of sodium azide?. Question: balance the following equation (using structural formulas). you will have to add reactant(s) and or product(s) to the left and or right hand sides in order to do this. based on your above answers, and your measured amounts, what was the limiting reactant for your reaction?. This ai generated tip is based on chegg's full solution. sign up to see more! start out by counting the total number of each type of atom on both sides of the equation. Balance each of the following equations according to the half reaction method: no2 (aq) mno2 (s) no3 (aq) (in base) 142 (aq) — mno4 (aq) mno2 (s) (in base) (c) br2 (l) so2 (g) →→ br (aq) so42 (aq) (in. there are 3 steps to solve this one. to balance this redox reaction in a basic solution using th 41.

Solved 5 Given The Following Equation A Balance The Chegg Question: 27) (4pts) balance the following chemical equation. al(no3)3 nas nano3 als 28) (3pts) automotive air bags inflate when sodium azide decomposes to its constituent elements: 2nan3 (s) 2na (s) 3n2 (8) how many moles of n2 are produced by the decomposition of 1.75 mol of sodium azide?. Question: balance the following equation (using structural formulas). you will have to add reactant(s) and or product(s) to the left and or right hand sides in order to do this. based on your above answers, and your measured amounts, what was the limiting reactant for your reaction?. This ai generated tip is based on chegg's full solution. sign up to see more! start out by counting the total number of each type of atom on both sides of the equation. Balance each of the following equations according to the half reaction method: no2 (aq) mno2 (s) no3 (aq) (in base) 142 (aq) — mno4 (aq) mno2 (s) (in base) (c) br2 (l) so2 (g) →→ br (aq) so42 (aq) (in. there are 3 steps to solve this one. to balance this redox reaction in a basic solution using th 41.
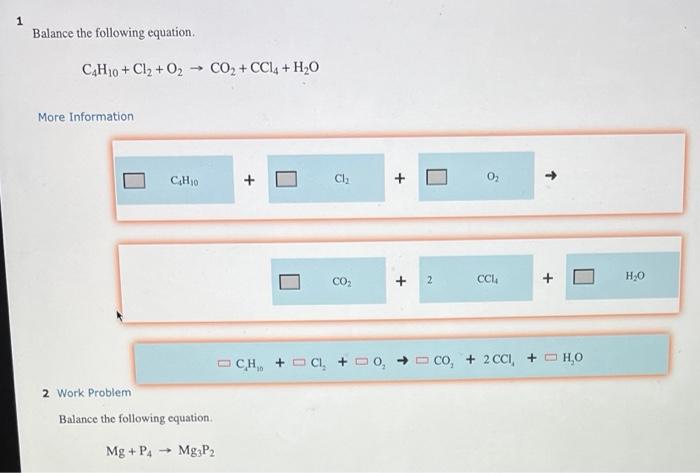
Solved 1 Balance The Following Equation Chegg This ai generated tip is based on chegg's full solution. sign up to see more! start out by counting the total number of each type of atom on both sides of the equation. Balance each of the following equations according to the half reaction method: no2 (aq) mno2 (s) no3 (aq) (in base) 142 (aq) — mno4 (aq) mno2 (s) (in base) (c) br2 (l) so2 (g) →→ br (aq) so42 (aq) (in. there are 3 steps to solve this one. to balance this redox reaction in a basic solution using th 41.
