Solved A Draw Two Linkage Isomers Co En Nh Scn 2 B Draw Geometric

Solved A Draw The Two Linkage Isomers Of Co Nh3 5scn 2 B Draw In the provided exercise, we have the compounds [co (nh 3) 5 cl] br and [co (nh 3) 5 br] cl, showcasing this isomerism through the exchange of chloride and bromide ions. Answer to solved 2 a) draw two linkage isomers [co (en)2nh3 scn]2 b) | chegg.
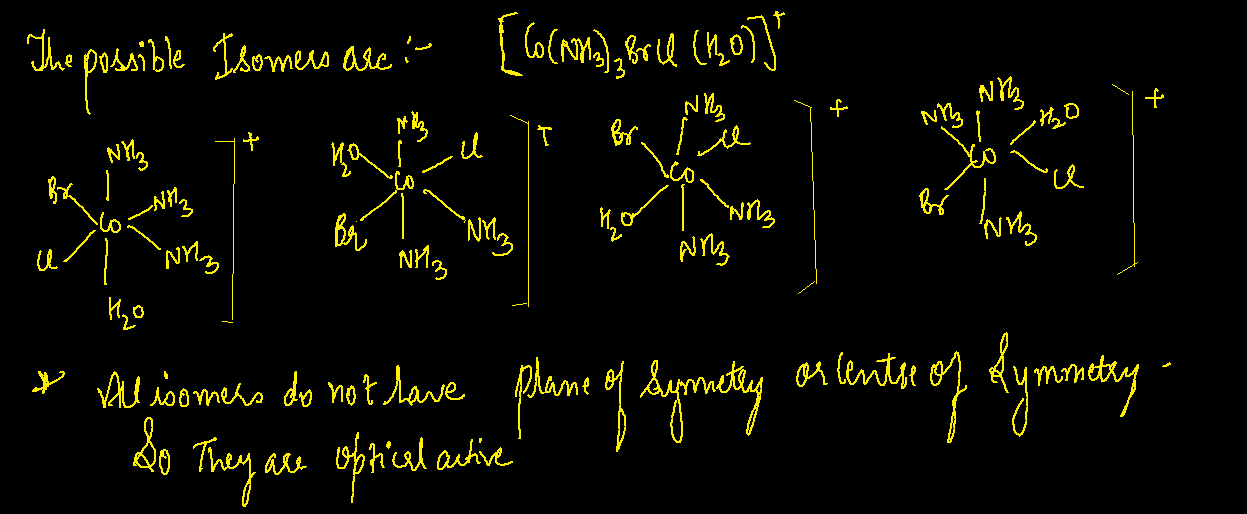
A Draw Two Linkage Isomers Co En 2nh3 Scn 2 B Draw Geometric Isome The structure can be represented as [co (nh3)3cl3]2 (fac cl) **geometric isomer 2**: [co (nh3)3cl3]2 (mer cl) co is in the center, surrounded by 3 nh3 ligands and 3 cl ligands in a meridional arrangement. B) draw geometric isomers for each above linkage isomers c) determine the number of geometric and optical isomers of [co (nh3)3brcl (h2o)] and draw all isomers. A) draw two linkage isomers [co (en)2 (nh3)scn]2 b) draw geometric isomers for each of the above linkage isomers c) determine the number of geometric and optical isomers of [co (nh3)2brcl (h2o)] and draw all isomers 3. In this case, we are looking at isomers of [c o(n h 3)5scn]2 where the thiocyanate ligand (scn ) can bind through either the sulfur (s) or nitrogen (n) atom to the central cobalt (co) atom.
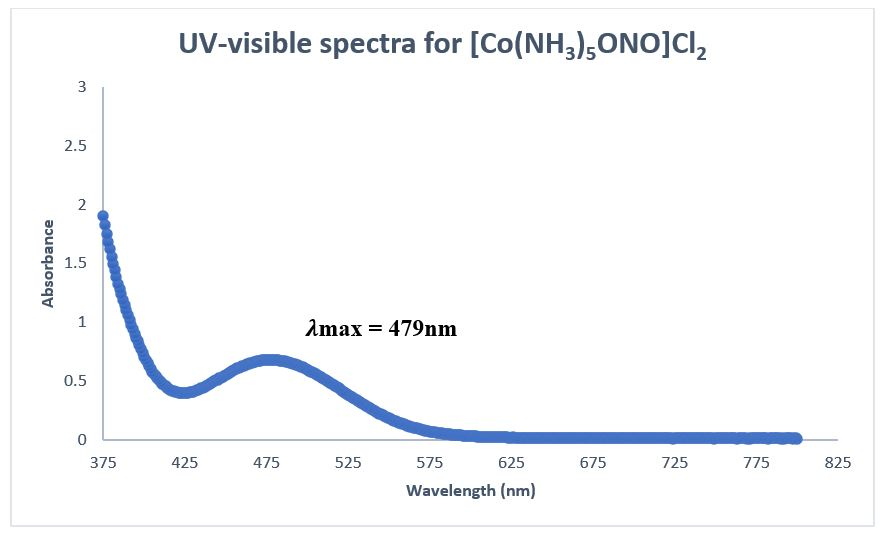
Solved The Two Linkage Isomers Of Co Nh3 5ono Cl2 Chegg A) draw two linkage isomers [co (en)2 (nh3)scn]2 b) draw geometric isomers for each of the above linkage isomers c) determine the number of geometric and optical isomers of [co (nh3)2brcl (h2o)] and draw all isomers 3. In this case, we are looking at isomers of [c o(n h 3)5scn]2 where the thiocyanate ligand (scn ) can bind through either the sulfur (s) or nitrogen (n) atom to the central cobalt (co) atom. Linkage isomerism occurs with ambidentate ligands that are capable of coordinating in more than one way. the best known cases involve the monodentate ligands: \ (scn^ ncs^ \) and \ (no 2^ ono^ \). the only difference is what atoms the molecular ligands bind to the central ion. Our expert help has broken down your problem into an easy to learn solution you can count on. question: 1. draw the two linkage isomers of [co (nh3)5scn]2 . b) draw the two geometric isomers of [co (nh3)3cl3]2 . c) two compounds with the formula co (nh3)5clbr can be prepared. use structural formulas to show how they differ. A) draw two linkage isomers [co (en)2nh3 scn]2 b) draw geometric isomers for each above linkage isomersc) determine the number of geometric and optical isomers of [co (nh3)3brcl (h2o)] and draw all isomers. Solution for (a) draw the two linkage isomers of [co (nh3 )5 scn]2 . (b) draw the two geometric isomers of $\left [\mathrm {co}\left (\mathrm {nh} {3}\right) {3} \mathrm {cl} {3}\right]^ {2 }$.

Solved Draw Two Linkage Isomers Of Mn Nh 3 5 No 2 2 Chegg Linkage isomerism occurs with ambidentate ligands that are capable of coordinating in more than one way. the best known cases involve the monodentate ligands: \ (scn^ ncs^ \) and \ (no 2^ ono^ \). the only difference is what atoms the molecular ligands bind to the central ion. Our expert help has broken down your problem into an easy to learn solution you can count on. question: 1. draw the two linkage isomers of [co (nh3)5scn]2 . b) draw the two geometric isomers of [co (nh3)3cl3]2 . c) two compounds with the formula co (nh3)5clbr can be prepared. use structural formulas to show how they differ. A) draw two linkage isomers [co (en)2nh3 scn]2 b) draw geometric isomers for each above linkage isomersc) determine the number of geometric and optical isomers of [co (nh3)3brcl (h2o)] and draw all isomers. Solution for (a) draw the two linkage isomers of [co (nh3 )5 scn]2 . (b) draw the two geometric isomers of $\left [\mathrm {co}\left (\mathrm {nh} {3}\right) {3} \mathrm {cl} {3}\right]^ {2 }$.

Solved 2 A Draw Two Linkage Isomers Co En 2nh3scn2 B Chegg A) draw two linkage isomers [co (en)2nh3 scn]2 b) draw geometric isomers for each above linkage isomersc) determine the number of geometric and optical isomers of [co (nh3)3brcl (h2o)] and draw all isomers. Solution for (a) draw the two linkage isomers of [co (nh3 )5 scn]2 . (b) draw the two geometric isomers of $\left [\mathrm {co}\left (\mathrm {nh} {3}\right) {3} \mathrm {cl} {3}\right]^ {2 }$.
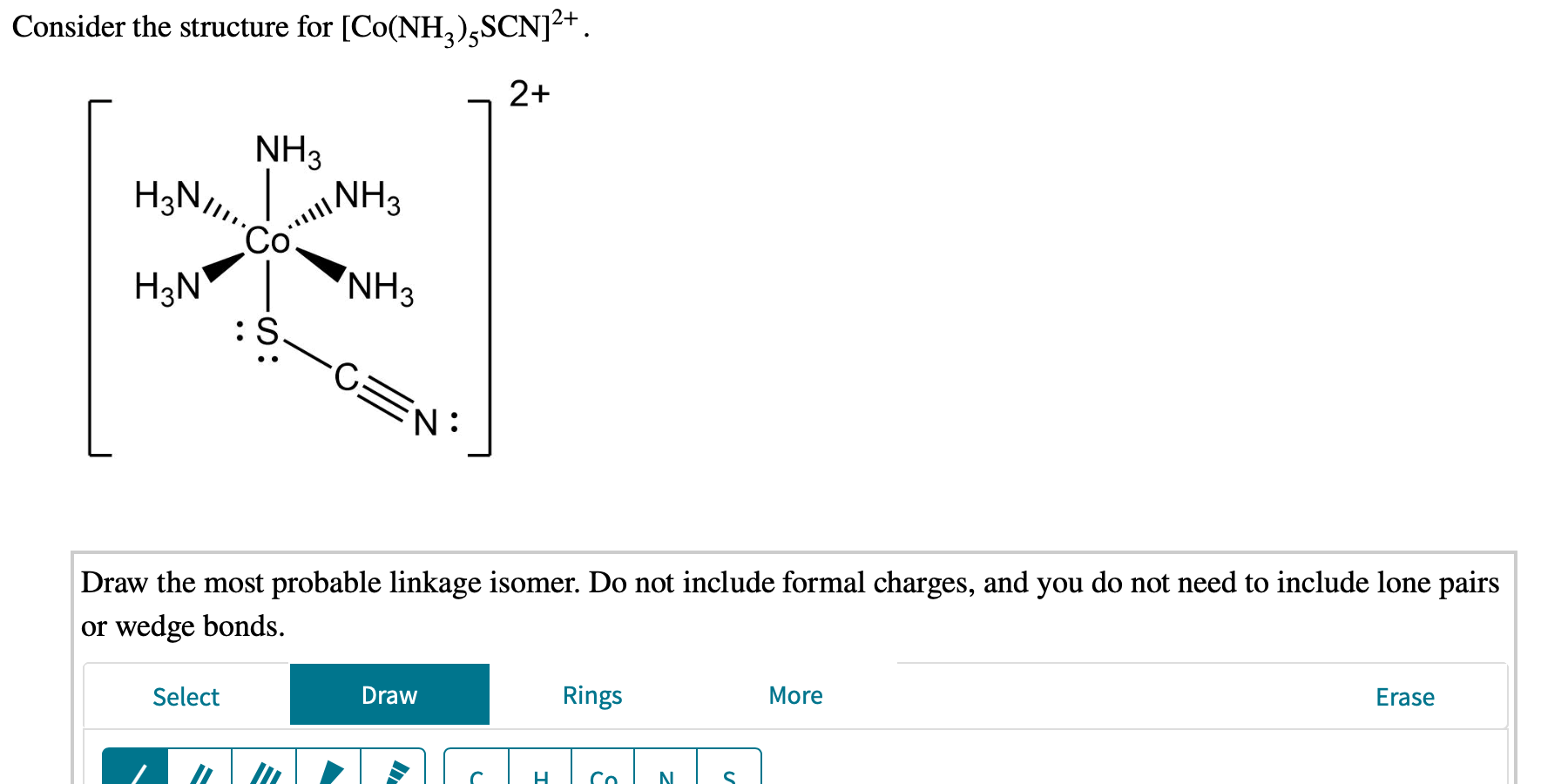
Solved Consider The Structure For Co Nh2 Scn 2 2 Chegg
Comments are closed.