Solved A Solution Contains 4 60 G Of Solute In 11 1 G Of Chegg

Solved A Solution Contains 1 50 G Of Solute In 13 0 G Of Chegg Here’s the best way to solve it. to start solving the problem, calculate the mass of the solution by adding the mass of the solute (4.60 g) to the mass of the solvent (11.1 g). hope y … a solution contains 4.60 g of solute in 11.1 g of solvent. what is the mass percent of the solute in the solution? mass percent. This calculator can solve problems on the molarity or molar concentration of a solute in a solution. first, it can calculate the molar concentration of a solute given a solute chemical formula, the mass of the solute and the volume of the solution.

Solved A Solution Contains 4 30 G Of Solute In 12 8 G Of Chegg What is the mass percent of the [chemistry] a solution contains 4.40 g of solute in 12.1 g of solvent. what is the mass percent of the solute in the solution? 36.3636 mass percent: incorrect. the mass percent of the solute in the solution is 26.67%. 😉 want a more accurate answer? get step by step solutions within seconds. To find the mass percent of the solute in the solution, you can follow these steps: the total mass of the solution is the sum of the mass of the solute and the mass of the solvent. given: mass of solute = 4.90 g and mass of solvent = 11.3 g. total mass of solution = 4.90 g 11.3 g = 16.20 g. Calculate the freezing point and boiling point of an antifreeze solution that is 50.0% by mass of ethylene glycol (hoch₂ch₂oh) in water. ethylene glycol is a nonelectrolyte. we have an expert written solution to this problem!. Mass percent = (mass of solute total mass of solution) x 100% mass percent = (4.50 g 16.00 g) x 100% mass percent = 28.125% therefore, the mass percent of the solute in the solution is 28.125%.

Solved A Solution Contains 3 10 G Of Solute In 10 2 G Of Chegg Calculate the freezing point and boiling point of an antifreeze solution that is 50.0% by mass of ethylene glycol (hoch₂ch₂oh) in water. ethylene glycol is a nonelectrolyte. we have an expert written solution to this problem!. Mass percent = (mass of solute total mass of solution) x 100% mass percent = (4.50 g 16.00 g) x 100% mass percent = 28.125% therefore, the mass percent of the solute in the solution is 28.125%. A 0.700 g sample of β‑galactosidase is dissolved in water to make 0.172 l of solution, and the osmotic pressure of the solution at 25 ∘c is found to be 0.867 mbar. Solved: ≌ a solution contains 4.90 g of solute in 12.1 g of solvent. what is the mass percent of t [chemistry] home study resources chemistry. Wolfram|alpha is a great tool for calculating the molarity, molality, mass fraction and amount fraction concentration of solutions. it also generates solution properties and preparation recipes. enter your queries using plain english. here are some examples illustrating how to ask for a dilution. Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. see answer.

Solved A Solution Contains 1 70 G Of Solute In 12 5 G Of Chegg A 0.700 g sample of β‑galactosidase is dissolved in water to make 0.172 l of solution, and the osmotic pressure of the solution at 25 ∘c is found to be 0.867 mbar. Solved: ≌ a solution contains 4.90 g of solute in 12.1 g of solvent. what is the mass percent of t [chemistry] home study resources chemistry. Wolfram|alpha is a great tool for calculating the molarity, molality, mass fraction and amount fraction concentration of solutions. it also generates solution properties and preparation recipes. enter your queries using plain english. here are some examples illustrating how to ask for a dilution. Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. see answer.

Solved A Solution Contains 1 00 G Of Solute In 14 1 G Of Chegg Wolfram|alpha is a great tool for calculating the molarity, molality, mass fraction and amount fraction concentration of solutions. it also generates solution properties and preparation recipes. enter your queries using plain english. here are some examples illustrating how to ask for a dilution. Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. see answer.
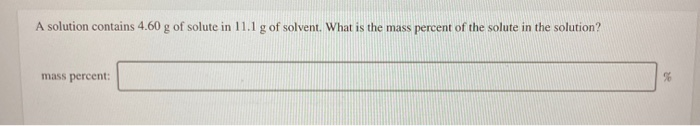
Solved A Solution Contains 4 60 G Of Solute In 11 1 G Of Chegg
Comments are closed.