Solved A Solution Is Prepared By Adding 0 10 Mol Of Chegg

Solved A Solution Prepared By Adding 0 300 Mol Glucose Chegg Question: a solution is prepared by adding 0.10 mol of potassium chloride, kcl , to 1.00 l of water. Given that the concentration of nh₃ is 3.0 m, the concentration of ni (nh₃)₆cl₂ is 0.20 m (as calculated initially), and k overall is 5.5 x 10^8, we can use this information to find the final concentrations of ni (nh₃)₆²⁺ and ni²⁺ after the reaction reaches equilibrium.

Solved A Solution Is Prepared By Adding 0 10 Mol Of Chegg Step 1: write down the balanced chemical equation for the reaction. the balanced chemical equation for the reaction is already provided in the exercise: ni2 (aq) 6nh3(aq)⇌ni (nh3)62 (aq) step 2: set up the equilibrium expression using the balanced equation. A solution is prepared by adding 0.10 mol of potassium acetate, kch 3coo , to 1.00 l of water. which statement about the solution is correct? the solution is acidic. the solution is basic. the concentrations of potassium ions and acetate ions will be identical. A solution is prepared by adding 0.10 mole of \mathrm {ni}\left (\mathrm {nh} {3}\right) {6} \mathrm {cl} {2} ni(nh3)6cl2 to 0.50 l of 3.0 m \mathrm {nh} {3}. This problem has been solved! you'll receive a detailed solution to help you master the concepts.
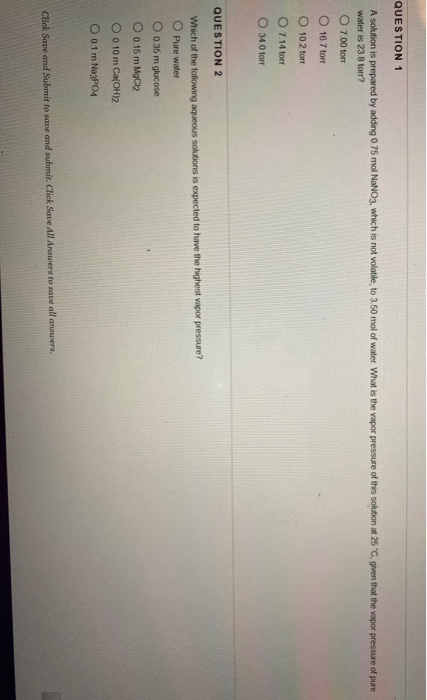
Solved Question 1 A Solution Is Prepared By Adding 0 75 Mol Chegg A solution is prepared by adding 0.10 mole of \mathrm {ni}\left (\mathrm {nh} {3}\right) {6} \mathrm {cl} {2} ni(nh3)6cl2 to 0.50 l of 3.0 m \mathrm {nh} {3}. This problem has been solved! you'll receive a detailed solution to help you master the concepts. Given that 1.5 moles of nh 3 are in a 0.50 l solution, its initial concentration is 3.0 m. as the reaction proceeds, the concentrations of species change and are affected, as shown in the ice table, requiring us to solve for equilibrium concentrations using the equation: x = 9.975 × 10 4 m. Verified answer and explanation explanation answer the answer is solved below. please go through it. Since sodium fluoride is a salt of a strong base (naoh) and a weak acid (hf), the solution will be basic. this is because the hydroxide ions (oh ) from the dissociation of water will react with the weak acid (hf) to form water and fluoride ions (f ). We can say that if 0.10 moles of it is dissolved in one liter of water, it will equal the number of either a plus or minus ion present in the evolution. we have provided statements about this solution.
Comments are closed.