Solved A Voltaic Cell Is Constructed From A Standard A%d0%b2 %d1%983 %d0%b2 %d1%98a%d0%b2 %d1%98 Chegg
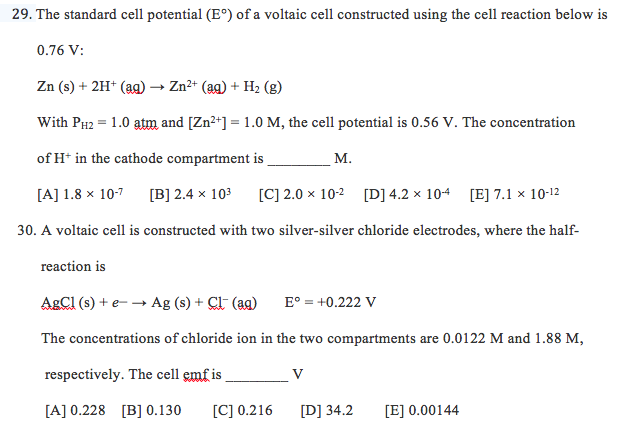
Solved The Standard Cell Potential E Of A Voltaic Cell Chegg A voltaic cell is constructed from a standard ag ∣ag half cell (e∘ red =0.799v) and a standard mn2 ∣mn half cell (ered ∘=−1.180v). the anode reaction is: the spontaneous cell reaction is: the cell voltage is v. 2. A voltaic cell is constructed from a standard ag | ag half cell (e°red = 0.799 v) and a standard fe3 | fe2 half cell (e°red = 0.771 v). (for all reactions below, use the smallest possible integer coefficients.

Solved A Voltaic Cell Is Constructed From A Standard Chegg A voltaic cell is constructed from a standard cr3 | cr half cell (e°red = 0.740 v) and a standard br2 | br half cell (e°red = 1.080 v). (for all reactions below, use the smallest possible integer coefficients. Step 1 2first, we need to identify the anode and cathode reactions. the anode is where oxidation occurs, and the cathode is where reduction occurs. since the standard reduction potential of f2 is higher than that of ag , f2 will be reduced at the cathode, and ag will be oxidized at the anode. In our voltaic cell exercise, the silver and iodine half reactions have standard reduction potentials of 0.80 v and 0.54 v, respectively. the silver reaction has a higher potential, indicating it is more likely to gain electrons and be reduced. A voltaic cell is constructed from a standard ag |ag half cell (e°red = 0.799v) and a standard cr3 |cr2 half cell (e°red = 0.410v). what is the spontaneous reaction that takes place, and what is the standard cell potential? your solution’s ready to go!.

Solved A Voltaic Cell Is Constructed From A Standard Chegg In our voltaic cell exercise, the silver and iodine half reactions have standard reduction potentials of 0.80 v and 0.54 v, respectively. the silver reaction has a higher potential, indicating it is more likely to gain electrons and be reduced. A voltaic cell is constructed from a standard ag |ag half cell (e°red = 0.799v) and a standard cr3 |cr2 half cell (e°red = 0.410v). what is the spontaneous reaction that takes place, and what is the standard cell potential? your solution’s ready to go!. The nernst equation is a fundamental concept in electrochemistry used to calculate the electromotive force (emf) of a voltaic cell when it is not under standard conditions. Identify which half cell will act as the anode by comparing the standard reduction potentials of the two half cells. Cell potential, denoted as e, is the driving force behind the electrical current produced by a voltaic cell. it is the difference in potential energy between the anode and cathode. A voltaic cell is constructed from a standard ag ag half cell (e°red 0.799v) and a standard cu cu2 half cell (e°red 0.153v). (use the lowest possible coefficients. use the pull down boxes to specify states such as (aq) or (s). if a box is not needed, leave it blank.).
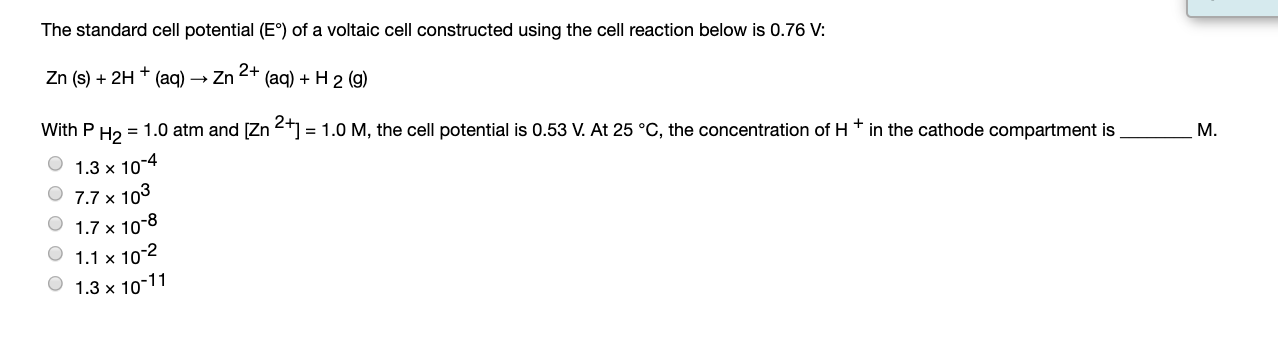
Solved The Standard Cell Potential Ec Of A Voltaic Cell Chegg The nernst equation is a fundamental concept in electrochemistry used to calculate the electromotive force (emf) of a voltaic cell when it is not under standard conditions. Identify which half cell will act as the anode by comparing the standard reduction potentials of the two half cells. Cell potential, denoted as e, is the driving force behind the electrical current produced by a voltaic cell. it is the difference in potential energy between the anode and cathode. A voltaic cell is constructed from a standard ag ag half cell (e°red 0.799v) and a standard cu cu2 half cell (e°red 0.153v). (use the lowest possible coefficients. use the pull down boxes to specify states such as (aq) or (s). if a box is not needed, leave it blank.).

Solved The Standard Cell Potential Eâ Of A Voltaic Cell Chegg Cell potential, denoted as e, is the driving force behind the electrical current produced by a voltaic cell. it is the difference in potential energy between the anode and cathode. A voltaic cell is constructed from a standard ag ag half cell (e°red 0.799v) and a standard cu cu2 half cell (e°red 0.153v). (use the lowest possible coefficients. use the pull down boxes to specify states such as (aq) or (s). if a box is not needed, leave it blank.).

Solved A Voltaic Cell Is Constructed From A Standard Chegg
Comments are closed.