Solved A Voltaic Cell Is Constructed From A Standard A13 Al Chegg
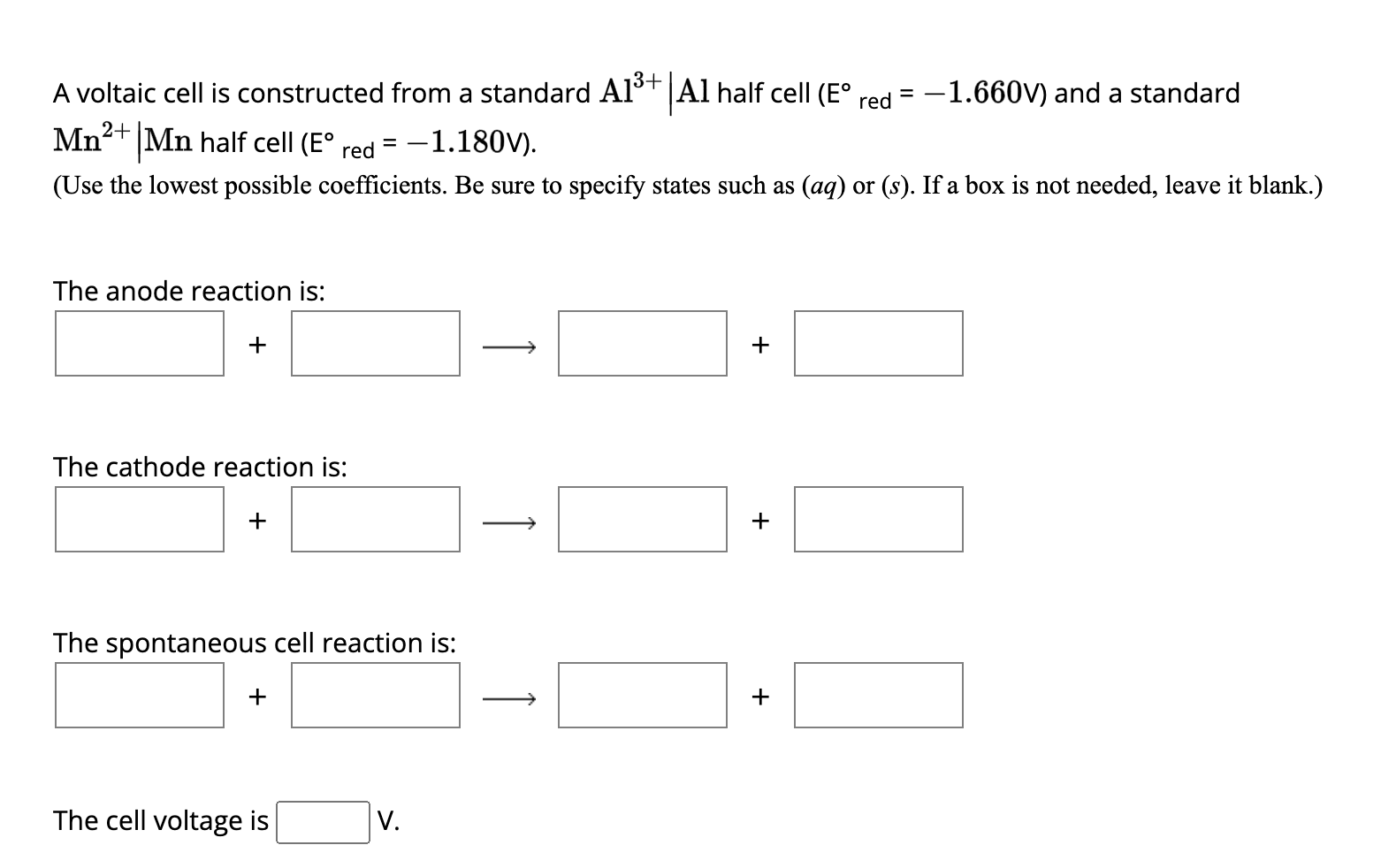
Solved A Voltaic Cell Is Constructed From A Standard Al3 Al Chegg A voltaic cell is constructed from a standard a13 |al half cell (e® red= 1.660v) and a standard cx |cr half cell (e® red = 0.740v). (use the lowest possible coefficients. be sure to specify states such as (aq) or (). if a box is not needed, leave it blank.). In a voltaic cell, oxidation occurs at the anode and reduction at the cathode, allowing for the flow of electrons through an external circuit. understanding the setup and function of these cells is crucial for analyzing their behavior under different conditions.

Solved A Voltaic Cell Is Constructed From A Standard Al Al Chegg A voltaic cell is constructed from a standard al 3 | al half cell (e° red = 1.660 v) and a standard sn answered step by step solved by verified expert marymount manhattan • chem. A voltaic cell is constructed from a standard h | h2 half cell (e°red = 0.000 v) and a standard f2 | f half cell (e°red = 2.870 v). (for all reactions below, use the smallest possible integer coefficients. Calculate the standard cell potential produced by a voltaic cell consisting of a gold electrode in contact with a solution of au3 ions and a silver electrode in contact with a solution of ag ions. The anode reaction is the oxidation half reaction, which is the one with the lower reduction potential. in this case, it's the ag | ag half cell. the cathode reaction is the reduction half reaction, which is the one with the higher reduction potential. in this case, it's the br2 | br half cell.
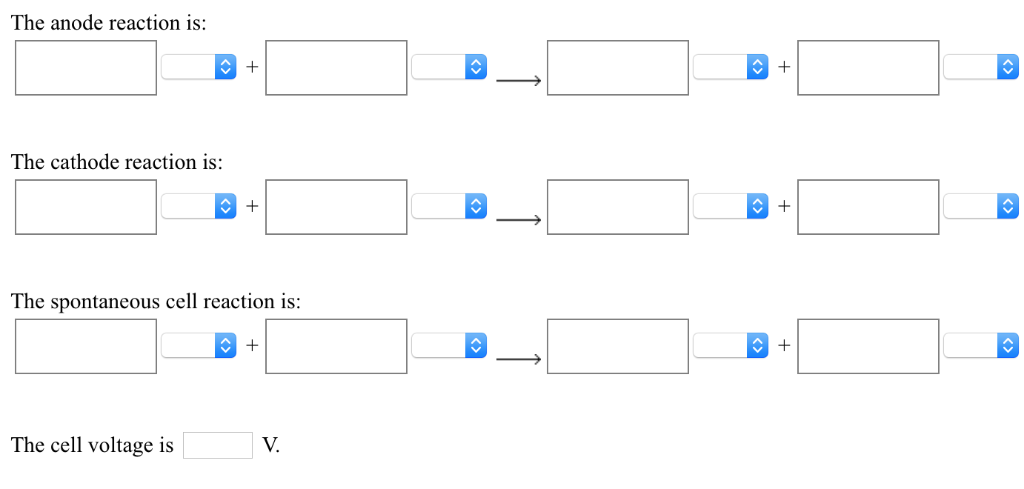
Solved A Voltaic Cell Is Constructed From A Standard Al3 Al Chegg Calculate the standard cell potential produced by a voltaic cell consisting of a gold electrode in contact with a solution of au3 ions and a silver electrode in contact with a solution of ag ions. The anode reaction is the oxidation half reaction, which is the one with the lower reduction potential. in this case, it's the ag | ag half cell. the cathode reaction is the reduction half reaction, which is the one with the higher reduction potential. in this case, it's the br2 | br half cell. Provide a detailed sketch of the voltaic cell, including as much details as you can including the direction of ion and electron flow, labeling of anode and cathode, and charges at the electrodes. This problem has been solved! you'll get a detailed solution from a subject matter expert that helps you learn core concepts. see answer. Here’s the best way to solve it. a voltaic cell is constructed from a standard a13 al half cell (eº red 1.660 v) and a standard zn2 zn half cell (e° red= 0.763v). (use the lowest possible coefficients. be sure to specify states such as (aq) or (s). if a box is not needed, leave it blank.). In a voltaic cell, the half cell with the lower reduction potential acts as the anode. so, the al3 |al half cell is the anode and the f2|f half cell is the cathode.
Comments are closed.