Solved At 30a C I The Ksp I Of Pbi2 I Is 3 2a 10 8 I What Is The Chegg
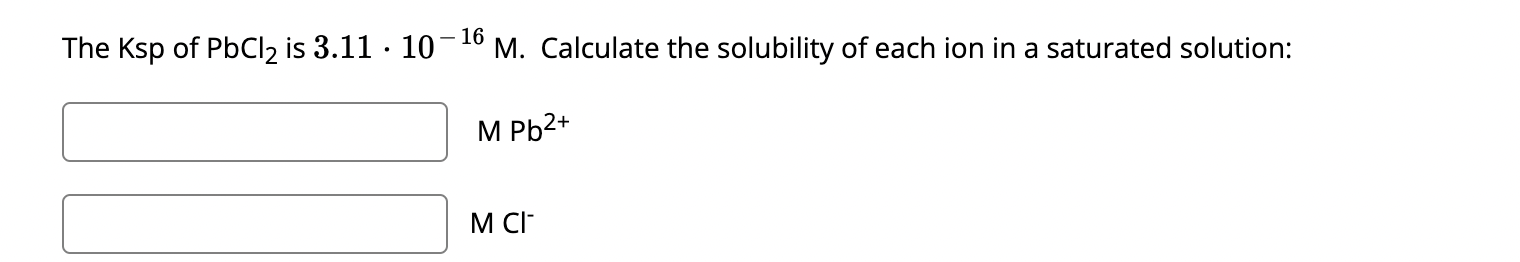
Solved The Ksp ï Of Pbcl2 ï Is 3 11 10 16m ï Calculate The Chegg Science chemistry chemistry questions and answers at 30°c the ksp of pbi2 is 3.2×10 8. what is the molar solubility of pbi2 in 1m solution of ki ?3.2×10 88×10 68×10 112×10 32×10 6 your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. see answer. The molar solubility of p bi 2 in a 1 m solution of ki at 30∘c is 3.2× 10−8. this is derived from the solubility product k sp expression, factoring in the concentration of iodide ions from ki.

Solved Given That The Ksp Value For Pbi2 Is 4 1 10 12 If Chegg The solubility product of pbi2 at 30°c is 1.4x10^ 8. calculate its solubility in 0.1m ki solution. Determine the ksp of pbi2 with this assignment. includes calculations, experimental data analysis, and equilibrium concepts. The discussion centers around the calculation of the solubility product constant (ksp) for lead iodide (pbi2). the user is confused about the ksp formula, which is given as ksp = [ca2 ] [io3 ]^2, indicating a misunderstanding of the components involved. We will establish the equilibrium by mixing the solutions of lead (ii) nitrate and potassium iodide to form a precipitate. once the mixture is prepared, the lead (ii) ions and the iodide ions that are dissolved in the solution will be in equilibrium with the solid lead (ii) iodide.

Solved Calculating Ksp From A Saturated Solution Of Pbl2 At Chegg The discussion centers around the calculation of the solubility product constant (ksp) for lead iodide (pbi2). the user is confused about the ksp formula, which is given as ksp = [ca2 ] [io3 ]^2, indicating a misunderstanding of the components involved. We will establish the equilibrium by mixing the solutions of lead (ii) nitrate and potassium iodide to form a precipitate. once the mixture is prepared, the lead (ii) ions and the iodide ions that are dissolved in the solution will be in equilibrium with the solid lead (ii) iodide. To determine the solubility of pbi₂ (lead (ii) iodide) in a 2.00 m solution of pb (no₃)₂ (lead (ii) nitrate), we need to consider the common ion effect and the solubility product constant (ksp). To find the molar solubility of pbi2 in a 1 m solution of ki, we'll use the concept of the solubility product constant (k sp). here's a step by step explanation:. (hint, to make this math youcan do in your head, 3.2×10 8 is 32×10 9.9×10 51.4×10 52×10 34×10 3. your solution’s ready to go! enhanced with ai, our expert help has broken down your problem into an easy to learn solution you can count on. question: at 30°c the ksp of pbi2 is 3.2×10 8. what is the molar solubility?. Lab manual for determining the ksp of pbi2 using spec 20. includes procedure, calculations, and prelab questions. chemistry experiment.
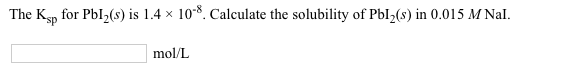
Solved The Ksp For Pbi S Is 1 4 10 8 Calculate The Chegg To determine the solubility of pbi₂ (lead (ii) iodide) in a 2.00 m solution of pb (no₃)₂ (lead (ii) nitrate), we need to consider the common ion effect and the solubility product constant (ksp). To find the molar solubility of pbi2 in a 1 m solution of ki, we'll use the concept of the solubility product constant (k sp). here's a step by step explanation:. (hint, to make this math youcan do in your head, 3.2×10 8 is 32×10 9.9×10 51.4×10 52×10 34×10 3. your solution’s ready to go! enhanced with ai, our expert help has broken down your problem into an easy to learn solution you can count on. question: at 30°c the ksp of pbi2 is 3.2×10 8. what is the molar solubility?. Lab manual for determining the ksp of pbi2 using spec 20. includes procedure, calculations, and prelab questions. chemistry experiment.
Comments are closed.