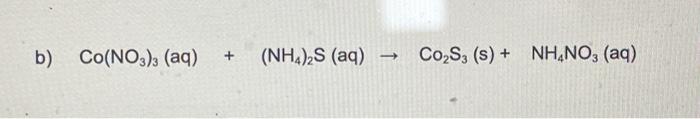
Solved B Co No3 3 Aq Nh4 2s Aq Co S3 S Chegg Tvc case nahco3 (aq) hcl (aq) h2o (l) nacl (aq) co2 (g) express your answer as a chemical equation. identify all of the phases in your answer. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. You can get 1 free, step by step answer each week. there’s just one step to solve this. example of the balancing of the given chemical equation.
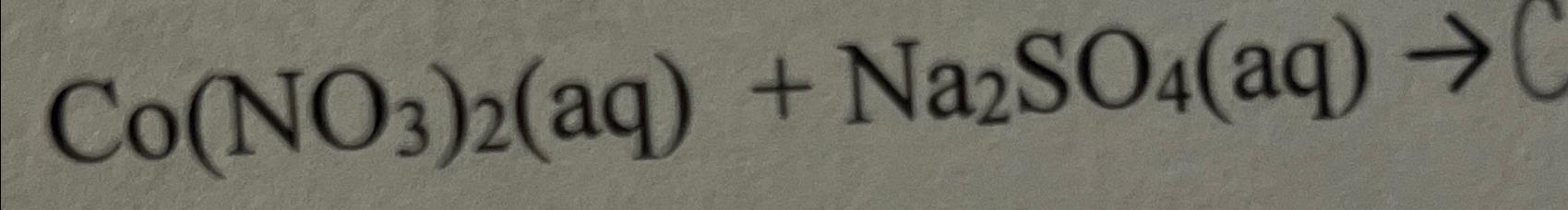
Solved Co No3 2 Aq Na2so4 Aq Chegg Here’s the best way to solve it. question 20 balance the following equation: co (no3)3 (aq) (nh4)2s (aq) → co2s3 (s) nh4no3 (aq) the stoichiometric coefficients, in the same order as the compounds written in the reaction, are a. 2,1,1,1 b. 2,2,1,4 oc. 2,3,1,4 d. 1,2,1,2 oe. 2.3.1,6. not the question you’re looking for?. Enter an equation of a chemical reaction and click 'balance'. the answer will appear below. always use the upper case for the first character in the element name and the lower case for the second character. examples: fe, au, co, br, c, o, n, f. compare: co cobalt and co carbon monoxide. Balance the reaction of co(no3)3 (nh4)2s = co2s3 nh4no3 using this chemical equation balancer!. In this video we'll balance the equation co (no3)3 (nh4)2s = co2s3 nh4no3 and provide the correct coefficients for each compound. more. to balance co (no3)3 (nh4)2s = co2s3.

Solved Part B Co No3 3 Aq Nh4 2s Aq Co2s3 S Nh No3 Chegg Balance the reaction of co(no3)3 (nh4)2s = co2s3 nh4no3 using this chemical equation balancer!. In this video we'll balance the equation co (no3)3 (nh4)2s = co2s3 nh4no3 and provide the correct coefficients for each compound. more. to balance co (no3)3 (nh4)2s = co2s3. Tro chemistry:a molecular approach 4th edition step by step solution to problem 111 in chapter 3. find answers and explanations to many question from your general chemistry textbook. Co(no3)3(aq) (nh4)2 s(aq)→co2 s3( s) nh4no3(aq) express your answer as a chemical equation including phases. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. 3 co(no 3 ) 2 (a q) 6 nh 4 s (a q) → co 2 s 3 (s) 6 nh 4 no 3 (a q) in this equation: \text{co(no} 3\text{)} 2 is in the aqueous phase (aq), indicating it is dissolved in water. \text{nh} 4\text{s} is also in the aqueous phase (aq). \text{co} 2\text{s} 3 is a solid (s), meaning it has precipitated out of solution. 7 3 3 if the sample being tested requires more extensive dilution than is provided by table 1, an intermediate dilution is prepared from 20 ml of sample diluted to 200 ml with odor free water use this dilution for the threshold determination multiply the t o n obtained by ten to correct for the intermediate dilution in rare cases more.

Solved Example Pb No3 2 Aq 2 Kl Aq 2 Kno3 Aq Chegg Tro chemistry:a molecular approach 4th edition step by step solution to problem 111 in chapter 3. find answers and explanations to many question from your general chemistry textbook. Co(no3)3(aq) (nh4)2 s(aq)→co2 s3( s) nh4no3(aq) express your answer as a chemical equation including phases. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. 3 co(no 3 ) 2 (a q) 6 nh 4 s (a q) → co 2 s 3 (s) 6 nh 4 no 3 (a q) in this equation: \text{co(no} 3\text{)} 2 is in the aqueous phase (aq), indicating it is dissolved in water. \text{nh} 4\text{s} is also in the aqueous phase (aq). \text{co} 2\text{s} 3 is a solid (s), meaning it has precipitated out of solution. 7 3 3 if the sample being tested requires more extensive dilution than is provided by table 1, an intermediate dilution is prepared from 20 ml of sample diluted to 200 ml with odor free water use this dilution for the threshold determination multiply the t o n obtained by ten to correct for the intermediate dilution in rare cases more.

Solved Part B Co No3 3 Aq Nh4 2s Aq Co2 S3 S Chegg 3 co(no 3 ) 2 (a q) 6 nh 4 s (a q) → co 2 s 3 (s) 6 nh 4 no 3 (a q) in this equation: \text{co(no} 3\text{)} 2 is in the aqueous phase (aq), indicating it is dissolved in water. \text{nh} 4\text{s} is also in the aqueous phase (aq). \text{co} 2\text{s} 3 is a solid (s), meaning it has precipitated out of solution. 7 3 3 if the sample being tested requires more extensive dilution than is provided by table 1, an intermediate dilution is prepared from 20 ml of sample diluted to 200 ml with odor free water use this dilution for the threshold determination multiply the t o n obtained by ten to correct for the intermediate dilution in rare cases more.
