Solved Be Sure To Answer All Parts Synthesize The Following Chegg

Solved Be Sure To Answer All Parts Synthesize The Following Chegg There are 2 steps to solve this one. be sure to answer all parts. synthesize the following compound from the given starting material. Overall, the synthetic pathway involves bromination of benzene, friedel crafts acylation, reduction of the ketone group, and reaction with a grignard reagent to synthesize 2 phenyl 2 propanol from benzene. each step must be carefully controlled to ensure the desired product is obtained.
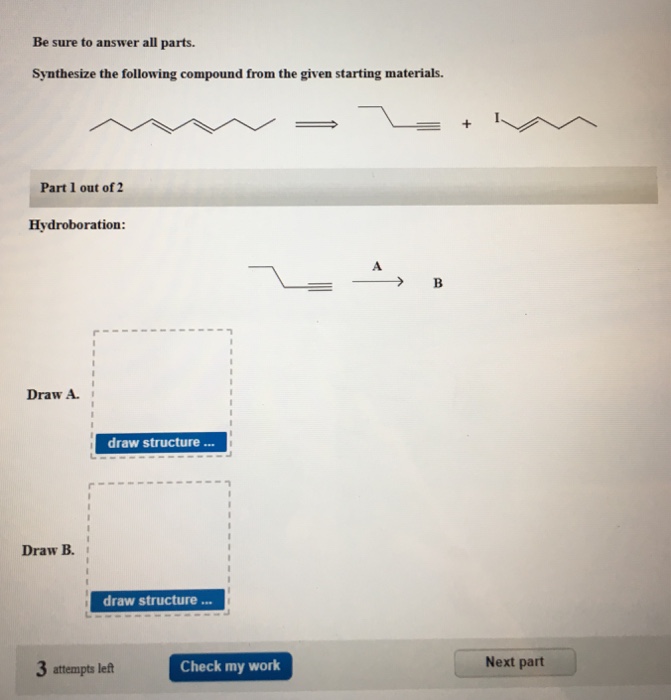
Solved Be Sure To Answer All Parts Synthesize The Following Chegg This problem has been solved! you'll receive a detailed solution to help you master the concepts. Instant text answer step 1 3 step 1: react 1 bromobutane with sodium cyanide (nacn) to form 1 cyanobutane (ch3ch2ch2cn). ch3ch2ch2ch2br nacn → ch3ch2ch2cn nabr step 2 3 step 2: react 1 cyanobutane with bromine (br2) to form 2 bromobutane 1 nitrile (ch3chbrch2cn). ch3ch2ch2cn br2 → ch3chbrch2cn hbr step 3 3. The synthesis of a compound from benzene typically involves steps such as electrophilic aromatic substitution to introduce functional groups, diazotization to form diazonium salts, and subsequent substitution reactions. the specific target compound will guide the choice of reagents and conditions. Be sure to answer all parts. show two methods to synthesize the alkene below: a one step method using a wittig reagent, and a two step method that forms a carbon carbon bond with an organometallic reagent in one of the steps.
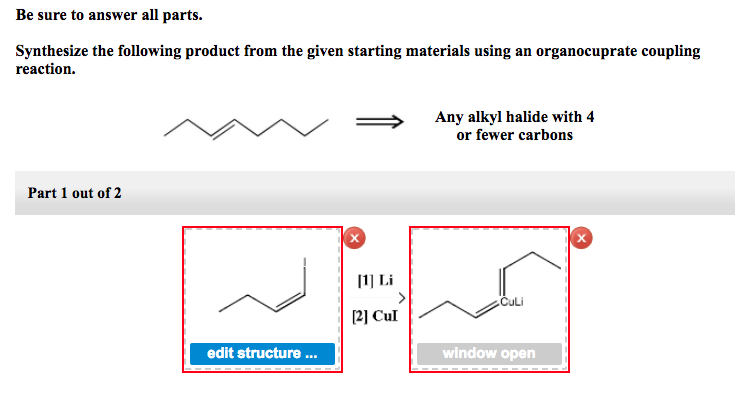
Solved Be Sure To Answer All Parts Synthesize The Following Chegg The synthesis of a compound from benzene typically involves steps such as electrophilic aromatic substitution to introduce functional groups, diazotization to form diazonium salts, and subsequent substitution reactions. the specific target compound will guide the choice of reagents and conditions. Be sure to answer all parts. show two methods to synthesize the alkene below: a one step method using a wittig reagent, and a two step method that forms a carbon carbon bond with an organometallic reagent in one of the steps. Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. see answer question: be sure to answer all parts. synthesize the following compound from benzene. use a diazonium salt as one of the synthetic intermediates. To synthesize an organometallic reagent for the desired compound starting from cyclohexanol and ethanol, the synthesis can be broken down into a few key steps:. Which of the following relationships must you consider before you are assigned to an audit client that could impact your independence? a.your spouse spousal equivalent's employment relationship. b.investments held by your non dependent siblings and. Be sure to answer all parts. synthesize the following compound from benzene. use a diazonium salt as one of the synthetic intermediates. draw the missing intermediates and select the correct reagents for the following.
Comments are closed.