Solved Calculate The Solubility Of Ag2cro4 A In Pure Water And B

Solved Calculate The Solubility Of Ag2cro4 A In Pure Water Chegg Here’s the best way to solve it. 1. solubility in pure water : ag2cro4 dissociate as, ag2cro4 2 ag^ cro4^ . s 2s s ksp = [ ag ]^2 [ cro4^ ] 1.2 * 10^ 12 = ( 2s)^2 ( s) 1.2 * 10^ 12 … not the question you’re looking for? post any question and get expert help quickly. Calculate the solubility of ag2cro4 in water. to calculate the solubility of ag2cro4 in water, we need to use the solubility product constant (ksp). the ksp of ag2cro4 is 1.1 x 10^ 12 at 25°c. first, we write the balanced chemical equation for the dissolution of ag2cro4 in water:.
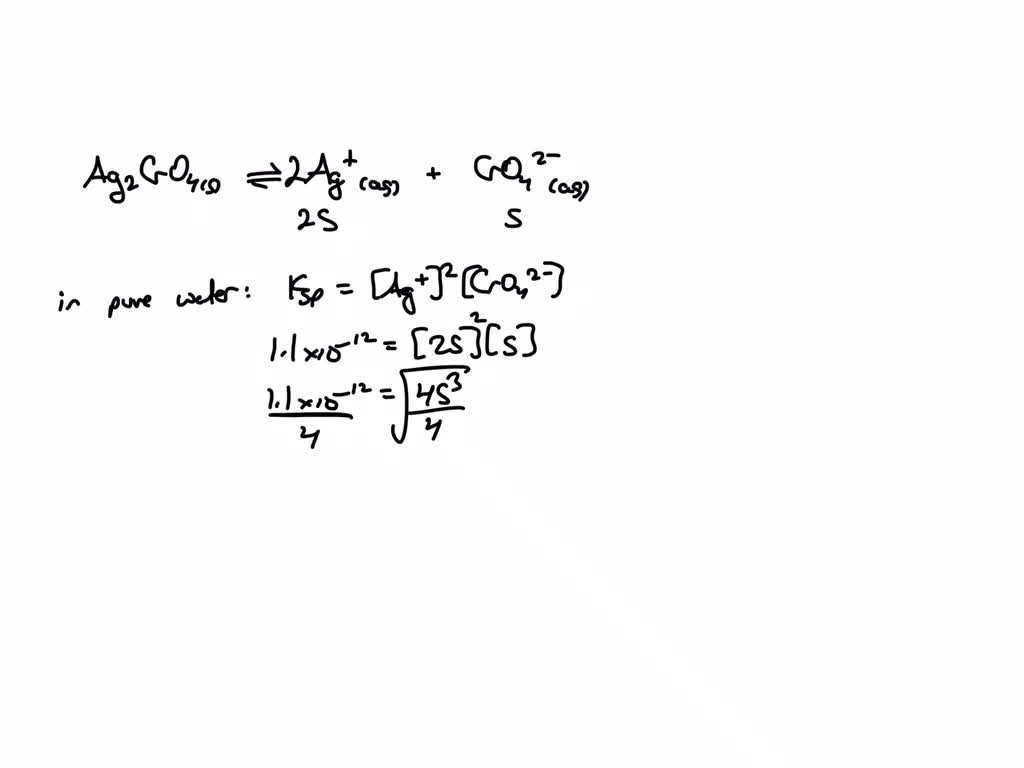
Solved Calculate The Solubility Of Ag2cro4 A In Pure Water And B To calculate the solubility of ag2cro4 in a saturated solution, we can use the boiling point elevation formula. the boiling point elevation is given by the formula: Δt b = i⋅k b⋅m where Δt b is the boiling point elevation, i is the van't hoff factor, k b is the ebullioscopic constant, and m is the molality of the solution. Video answer: turning with our solubility equilibrium for ag2cro4. equilibrium with 2ag , cro4 2 . this would be 2s, s. so in pure water, ksp equal to ag plus squared cro4 2 . ksp here. calculate the solubility. i'm going to look up the ksp here. To solve this problem, we need to calculate the molar solubility of silver chromate (ag2cro4) in two different scenarios: pure water and in a solution already containing potassium chromate (k2cro4). Calculate the molar solubility of agcro4 (s) for b. everything stays the same except (cro4^=) = s 0.1. substitute into ksp and solve for s. you can make the simplifying assumption that s 0.1 = 0.1. if you do, to avoid solving a quadratic equation, then check at the end to make sure s 0.10 = 0.1.

Solved Calculate The Solubility Of Ag2cro4 In A Water B Chegg To solve this problem, we need to calculate the molar solubility of silver chromate (ag2cro4) in two different scenarios: pure water and in a solution already containing potassium chromate (k2cro4). Calculate the molar solubility of agcro4 (s) for b. everything stays the same except (cro4^=) = s 0.1. substitute into ksp and solve for s. you can make the simplifying assumption that s 0.1 = 0.1. if you do, to avoid solving a quadratic equation, then check at the end to make sure s 0.10 = 0.1. Solution the solubility product constant, ksp, is a measure of the solubility of a compound. it is calculated by multiplying the concentrations of the ions that make up the compound. the compound ag2cro4 dissociates in water as follows: ag2cro4(s) ⇌ 2ag (aq) cro4^2 (aq). To determine the relative solubility of silver chromate (ag2cro4) in three different solvents, we will analyze each case step by step based on the common ion effect and the solubility product constant (ksp). 1) a) we know ag2cro4 dissociate in water as ag2cro4 (s) > 2ag (aq) cro42 (aq) so here the solubility product (ksp) can be calculated as ksp = [ag (aq)]2 * [cro42 (aq)] agian we know ksp for ag2cro4 = 1.2 x 10 12 now lets say …. The molar solubility of ag2cro4 in water, with a ksp of 8 x 10^ 12, is calculated based on the dissolution equation and the concentrations of ions in a saturated solution.

Solved Calculate The Solubility Of Ag2cro4 In Water At 25тишc Chegg Solution the solubility product constant, ksp, is a measure of the solubility of a compound. it is calculated by multiplying the concentrations of the ions that make up the compound. the compound ag2cro4 dissociates in water as follows: ag2cro4(s) ⇌ 2ag (aq) cro4^2 (aq). To determine the relative solubility of silver chromate (ag2cro4) in three different solvents, we will analyze each case step by step based on the common ion effect and the solubility product constant (ksp). 1) a) we know ag2cro4 dissociate in water as ag2cro4 (s) > 2ag (aq) cro42 (aq) so here the solubility product (ksp) can be calculated as ksp = [ag (aq)]2 * [cro42 (aq)] agian we know ksp for ag2cro4 = 1.2 x 10 12 now lets say …. The molar solubility of ag2cro4 in water, with a ksp of 8 x 10^ 12, is calculated based on the dissolution equation and the concentrations of ions in a saturated solution.
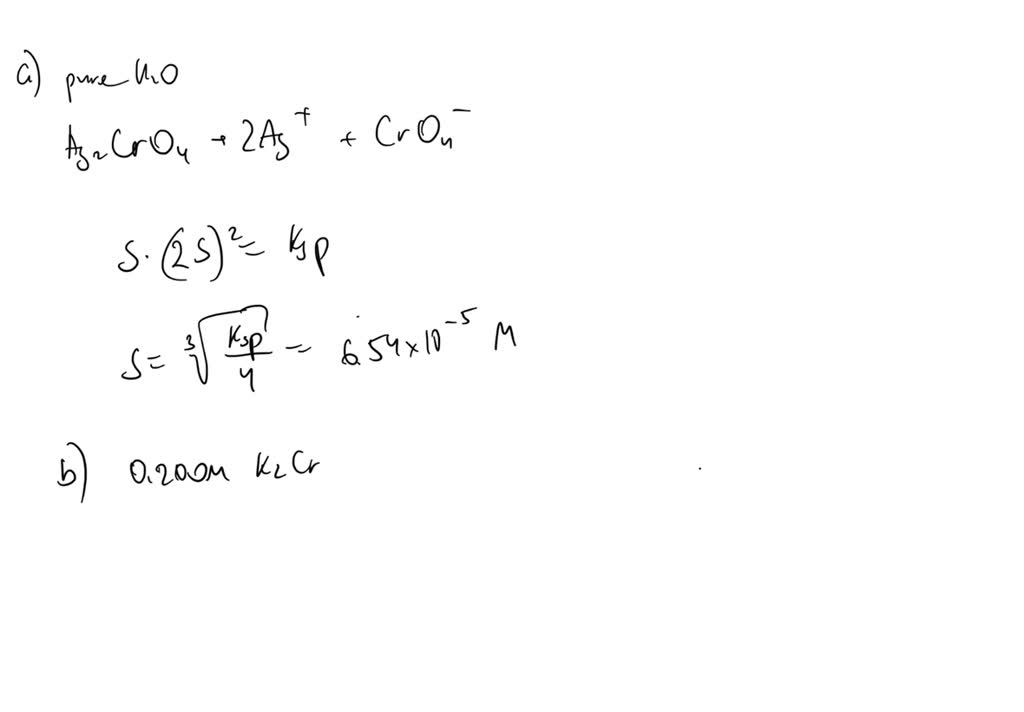
Solved Calculate The Molar Solubility Of Ag2cro4 Ksp 1 12ã 10 12 1) a) we know ag2cro4 dissociate in water as ag2cro4 (s) > 2ag (aq) cro42 (aq) so here the solubility product (ksp) can be calculated as ksp = [ag (aq)]2 * [cro42 (aq)] agian we know ksp for ag2cro4 = 1.2 x 10 12 now lets say …. The molar solubility of ag2cro4 in water, with a ksp of 8 x 10^ 12, is calculated based on the dissolution equation and the concentrations of ions in a saturated solution.
Comments are closed.