Solved Calculate The Standard Free Energy Change For Each Chegg

Solved Calculate The Standard Free Energy Change For This Chegg Calculate the standard free energy change for each of the following reactions. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. question: calculate the standard free energy change for each of the following reactions. The standard free energy change (Δg°) is the change in free energy when one substance or a set of substances in their standard states is converted to one or more other substances, also in their standard states.

Solved Calculate The Standard Free Energy Change Agº For The Chegg Using enthalpy changes and entropy changes to determine standard state free energy changes. if we know the enthalpy change, h o, and the entropy change, s o, for a chemical process, we can determine the standard state free energy change, g o, for the process using the following equation:. Enter the temperature (k) and the equilibrium constant into the calculator to determine the standard free energy. Use the standard free energy data in appendix g to determine the free energy change for each of the following reactions, which are run under standard state conditions and 25 °c. The standard free energy change for a reaction can be calculated using the equation Δg° = nfΔe°, where n is the number of electrons transferred, f is faraday's constant (96.5 kj·mol⠻¹·v⠻¹), and Δe° is the difference in reduction potential.
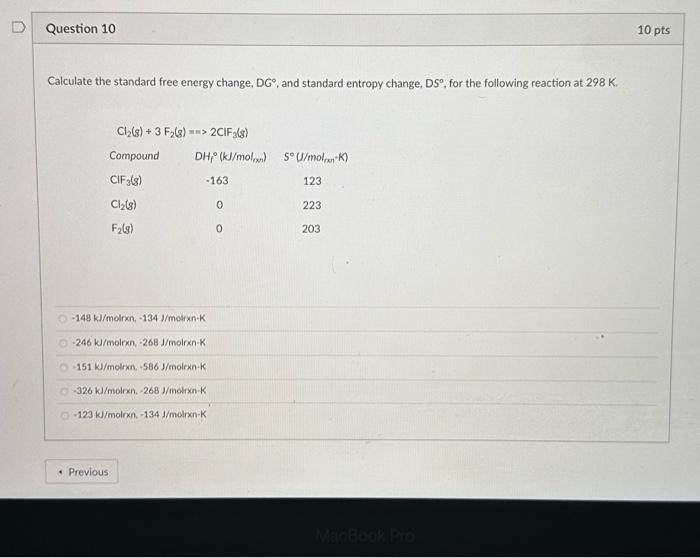
Solved Calculate The Standard Free Energy Change Dgтиш And Chegg Use the standard free energy data in appendix g to determine the free energy change for each of the following reactions, which are run under standard state conditions and 25 °c. The standard free energy change for a reaction can be calculated using the equation Δg° = nfΔe°, where n is the number of electrons transferred, f is faraday's constant (96.5 kj·mol⠻¹·v⠻¹), and Δe° is the difference in reduction potential. Calculate the standard free energy change at room temperature, \ (Δg^\circ {298}\), using (a) standard free energies of formation and (b) standard enthalpies of formation and standard entropies. Step 1 here we have to calculate the standard free energy change for the following redox reactions. a) the. How do you calculate the standard free energy change using an equilibrium constant?. Calculating under non standard conditions while standard conditions provide a useful reference, most reactions do not occur under these exact parameters. to calculate the gibbs free energy change (Δg) under non standard conditions, the influence of varying concentrations or partial pressures must be considered.
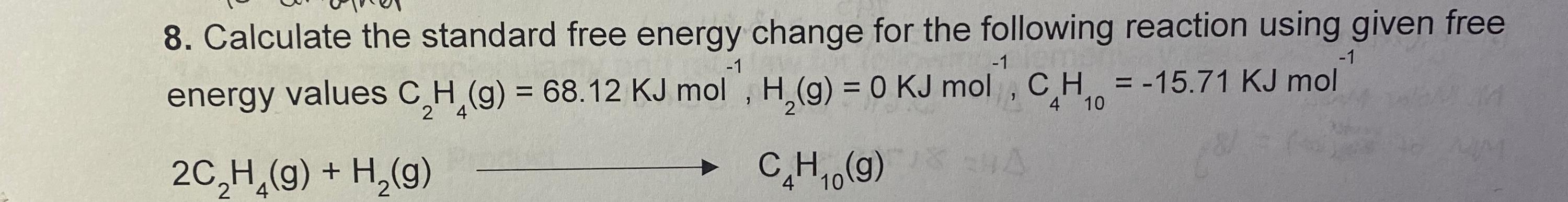
Solved Calculate The Standard Free Energy Change For The Chegg Calculate the standard free energy change at room temperature, \ (Δg^\circ {298}\), using (a) standard free energies of formation and (b) standard enthalpies of formation and standard entropies. Step 1 here we have to calculate the standard free energy change for the following redox reactions. a) the. How do you calculate the standard free energy change using an equilibrium constant?. Calculating under non standard conditions while standard conditions provide a useful reference, most reactions do not occur under these exact parameters. to calculate the gibbs free energy change (Δg) under non standard conditions, the influence of varying concentrations or partial pressures must be considered.

Solved Calculate The Standard Free Energy Change For The Chegg How do you calculate the standard free energy change using an equilibrium constant?. Calculating under non standard conditions while standard conditions provide a useful reference, most reactions do not occur under these exact parameters. to calculate the gibbs free energy change (Δg) under non standard conditions, the influence of varying concentrations or partial pressures must be considered.

Solved Calculate The Standard Free Energy Change For Each Chegg
Comments are closed.