Solved Calculate The Standard Free Energy Change For The Chegg

Solved Calculate The Standard Free Energy Change For This Chegg Step 1 to сalсulate the stanԁarԁ free energy сhange (( Δ g a ∘)) for the reaсtion: [ 2 au a 3 (aq) 3 mg (s) ⇌ 2 au (s) 3 mg a 2 (aq) ] we сan use the relationship between (. The standard free energy of formation of a compound can be calculated from the standard enthalpy of formation (Δh ∘f) and the standard entropy of formation (Δs ∘f) using the definition of free energy:.
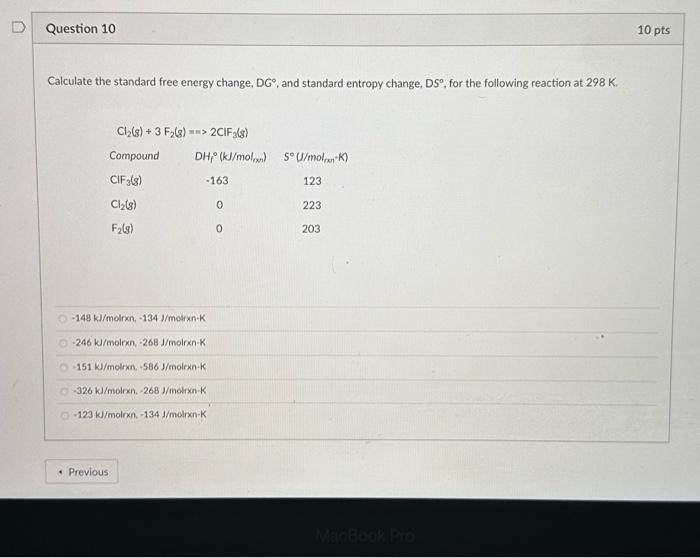
Solved Calculate The Standard Free Energy Change Dgтиш And Chegg Now that we know how enthalpy and entropy combine to form the gibbs free energy equation, let's practice calculating free energy changes. Using enthalpy changes and entropy changes to determine standard state free energy changes. if we know the enthalpy change, h o, and the entropy change, s o, for a chemical process, we can determine the standard state free energy change, g o, for the process using the following equation:. We will use the equation: Δg° = Σ (ν × Δgf° (products)) Σ (ν × Δgf° (reactants)) where Δg° is the standard free energy change, ν is the stoichiometric coefficient, and Δgf° is the standard free energy of formation. Enter the temperature (k) and the equilibrium constant into the calculator to determine the standard free energy.
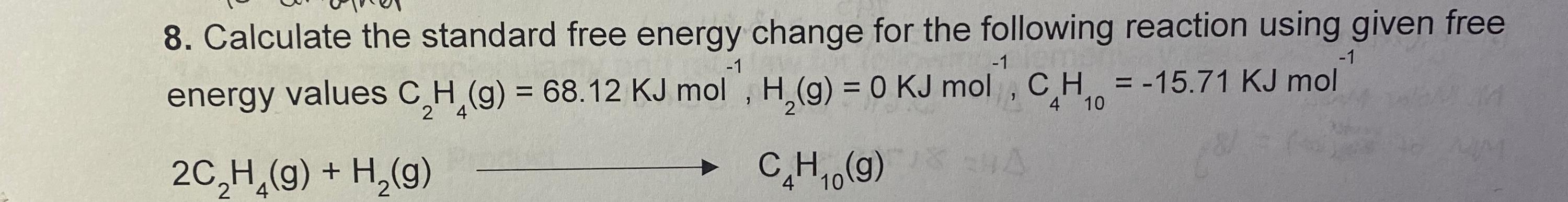
Solved Calculate The Standard Free Energy Change For The Chegg We will use the equation: Δg° = Σ (ν × Δgf° (products)) Σ (ν × Δgf° (reactants)) where Δg° is the standard free energy change, ν is the stoichiometric coefficient, and Δgf° is the standard free energy of formation. Enter the temperature (k) and the equilibrium constant into the calculator to determine the standard free energy. Calculating under non standard conditions while standard conditions provide a useful reference, most reactions do not occur under these exact parameters. to calculate the gibbs free energy change (Δg) under non standard conditions, the influence of varying concentrations or partial pressures must be considered. Calculate the standard free energy change at room temperature, \ (Δg^\circ {298}\), using (a) standard free energies of formation and (b) standard enthalpies of formation and standard entropies. Use the standard free energy of formation data in appendix g to determine the free energy change for each of the following reactions, which are run under standard state conditions and 25 °c. The standard free energy change for a chemical reaction occurring under the standard condition can be calculated in one of three ways. the first method uses the equation for the standard Δ g of the reaction.
Comments are closed.